| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 6466820 | Chemical Engineering Science | 2017 | 10 Pages |
â¢Model soot (Printex U) oxidation by NO2 and O2 in the presence of H2O.â¢Soot oxidation initiated by NO2 at low Temperatures.â¢Synergistic effect between NO2 and O2 on soot combustion.â¢H2O promotion on soot oxidation.â¢Dependence of the kinetic parameters on carbon conversion.
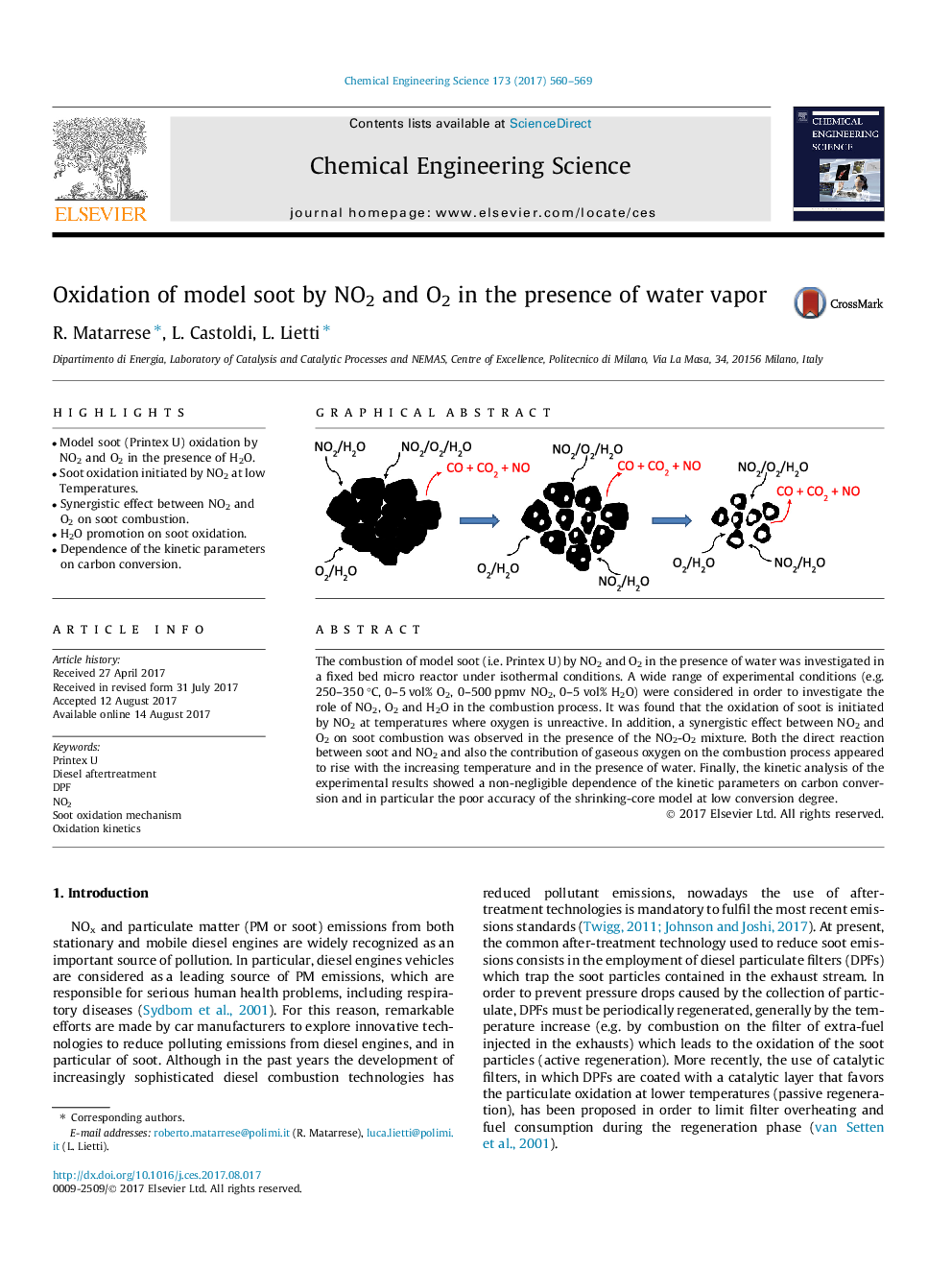
The combustion of model soot (i.e. Printex U) by NO2 and O2 in the presence of water was investigated in a fixed bed micro reactor under isothermal conditions. A wide range of experimental conditions (e.g. 250-350 °C, 0-5 vol% O2, 0-500 ppmv NO2, 0-5 vol% H2O) were considered in order to investigate the role of NO2, O2 and H2O in the combustion process. It was found that the oxidation of soot is initiated by NO2 at temperatures where oxygen is unreactive. In addition, a synergistic effect between NO2 and O2 on soot combustion was observed in the presence of the NO2-O2 mixture. Both the direct reaction between soot and NO2 and also the contribution of gaseous oxygen on the combustion process appeared to rise with the increasing temperature and in the presence of water. Finally, the kinetic analysis of the experimental results showed a non-negligible dependence of the kinetic parameters on carbon conversion and in particular the poor accuracy of the shrinking-core model at low conversion degree.
Graphical abstractDownload high-res image (130KB)Download full-size image