| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 6467193 | Chemical Engineering Science | 2017 | 10 Pages |
â¢Absorption heat was estimated using specific heat capacity and temperature.â¢Sensible heat of AMP-PZ-MEA blends is significantly lower than that of MEA.â¢Vaporization heat of the amine blends is lower than MEA.â¢Heat duty of the AMP-PZ-MEA blends is substantially lower than that of MEA.â¢A developed model accurately predicted AMP-PZ-MEA specific heat capacity.
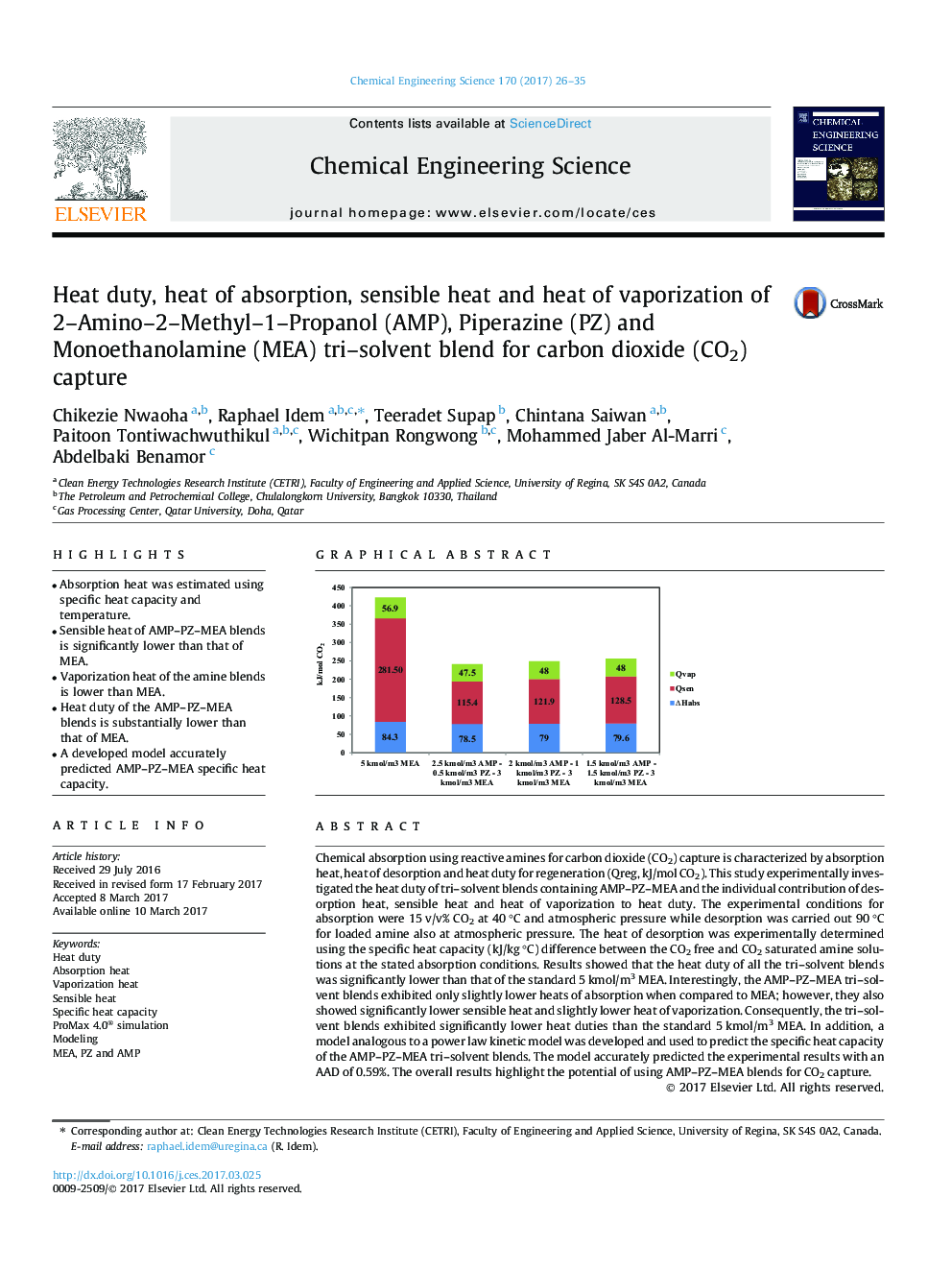
Chemical absorption using reactive amines for carbon dioxide (CO2) capture is characterized by absorption heat, heat of desorption and heat duty for regeneration (Qreg, kJ/mol CO2). This study experimentally investigated the heat duty of tri-solvent blends containing AMP-PZ-MEA and the individual contribution of desorption heat, sensible heat and heat of vaporization to heat duty. The experimental conditions for absorption were 15 v/v% CO2 at 40 °C and atmospheric pressure while desorption was carried out 90 °C for loaded amine also at atmospheric pressure. The heat of desorption was experimentally determined using the specific heat capacity (kJ/kg °C) difference between the CO2 free and CO2 saturated amine solutions at the stated absorption conditions. Results showed that the heat duty of all the tri-solvent blends was significantly lower than that of the standard 5 kmol/m3 MEA. Interestingly, the AMP-PZ-MEA tri-solvent blends exhibited only slightly lower heats of absorption when compared to MEA; however, they also showed significantly lower sensible heat and slightly lower heat of vaporization. Consequently, the tri-solvent blends exhibited significantly lower heat duties than the standard 5 kmol/m3 MEA. In addition, a model analogous to a power law kinetic model was developed and used to predict the specific heat capacity of the AMP-PZ-MEA tri-solvent blends. The model accurately predicted the experimental results with an AAD of 0.59%. The overall results highlight the potential of using AMP-PZ-MEA blends for CO2 capture.
Graphical abstractDownload high-res image (118KB)Download full-size image