| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 679463 | Bioresource Technology | 2015 | 6 Pages |
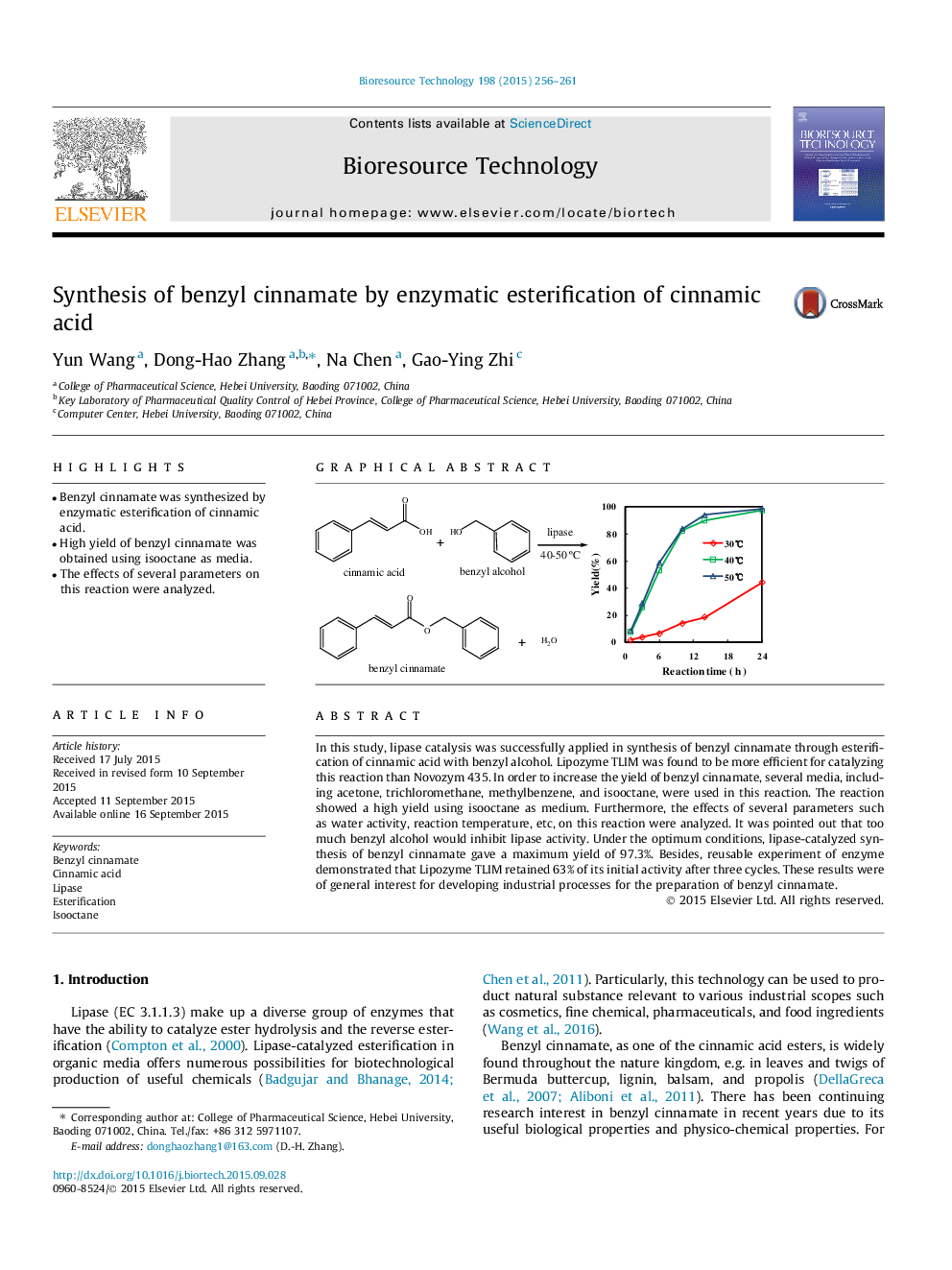
•Benzyl cinnamate was synthesized by enzymatic esterification of cinnamic acid.•High yield of benzyl cinnamate was obtained using isooctane as media.•The effects of several parameters on this reaction were analyzed.
In this study, lipase catalysis was successfully applied in synthesis of benzyl cinnamate through esterification of cinnamic acid with benzyl alcohol. Lipozyme TLIM was found to be more efficient for catalyzing this reaction than Novozym 435. In order to increase the yield of benzyl cinnamate, several media, including acetone, trichloromethane, methylbenzene, and isooctane, were used in this reaction. The reaction showed a high yield using isooctane as medium. Furthermore, the effects of several parameters such as water activity, reaction temperature, etc, on this reaction were analyzed. It was pointed out that too much benzyl alcohol would inhibit lipase activity. Under the optimum conditions, lipase-catalyzed synthesis of benzyl cinnamate gave a maximum yield of 97.3%. Besides, reusable experiment of enzyme demonstrated that Lipozyme TLIM retained 63% of its initial activity after three cycles. These results were of general interest for developing industrial processes for the preparation of benzyl cinnamate.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide