| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 679609 | Bioresource Technology | 2015 | 4 Pages |
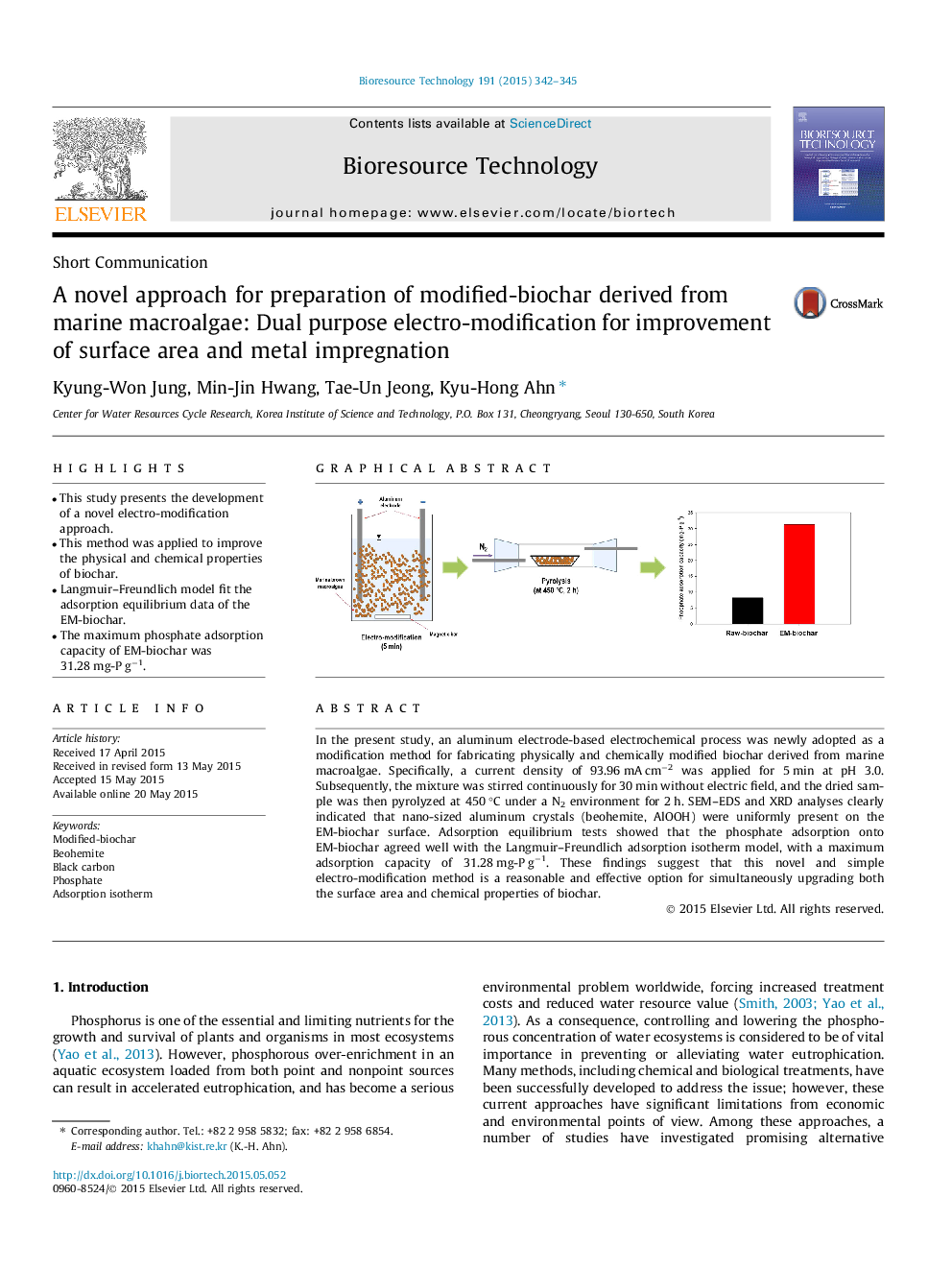
•This study presents the development of a novel electro-modification approach.•This method was applied to improve the physical and chemical properties of biochar.•Langmuir–Freundlich model fit the adsorption equilibrium data of the EM-biochar.•The maximum phosphate adsorption capacity of EM-biochar was 31.28 mg-P g−1.
In the present study, an aluminum electrode-based electrochemical process was newly adopted as a modification method for fabricating physically and chemically modified biochar derived from marine macroalgae. Specifically, a current density of 93.96 mA cm−2 was applied for 5 min at pH 3.0. Subsequently, the mixture was stirred continuously for 30 min without electric field, and the dried sample was then pyrolyzed at 450 °C under a N2 environment for 2 h. SEM–EDS and XRD analyses clearly indicated that nano-sized aluminum crystals (beohemite, AlOOH) were uniformly present on the EM-biochar surface. Adsorption equilibrium tests showed that the phosphate adsorption onto EM-biochar agreed well with the Langmuir–Freundlich adsorption isotherm model, with a maximum adsorption capacity of 31.28 mg-P g−1. These findings suggest that this novel and simple electro-modification method is a reasonable and effective option for simultaneously upgrading both the surface area and chemical properties of biochar.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide