| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 7672253 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 7 Pages |
Abstract
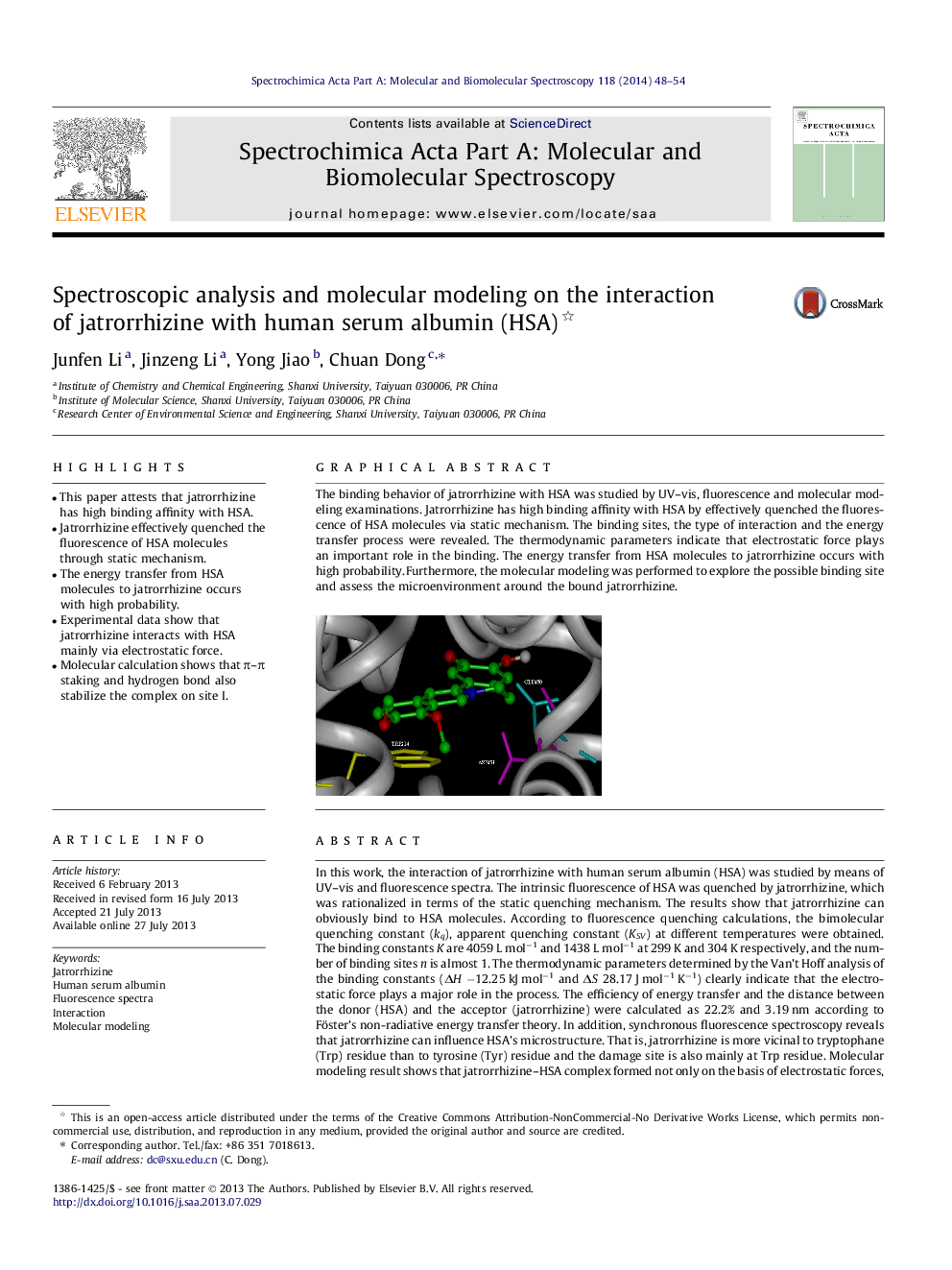
The binding behavior of jatrorrhizine with HSA was studied by UV-vis, fluorescence and molecular modeling examinations. Jatrorrhizine has high binding affinity with HSA by effectively quenched the fluorescence of HSA molecules via static mechanism. The binding sites, the type of interaction and the energy transfer process were revealed. The thermodynamic parameters indicate that electrostatic force plays an important role in the binding. The energy transfer from HSA molecules to jatrorrhizine occurs with high probability. Furthermore, the molecular modeling was performed to explore the possible binding site and assess the microenvironment around the bound jatrorrhizine.
Related Topics
Physical Sciences and Engineering
Chemistry
Analytical Chemistry
Authors
Junfen Li, Jinzeng Li, Yong Jiao, Chuan Dong,