| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 7842432 | Journal of Molecular Liquids | 2018 | 8 Pages |
Abstract
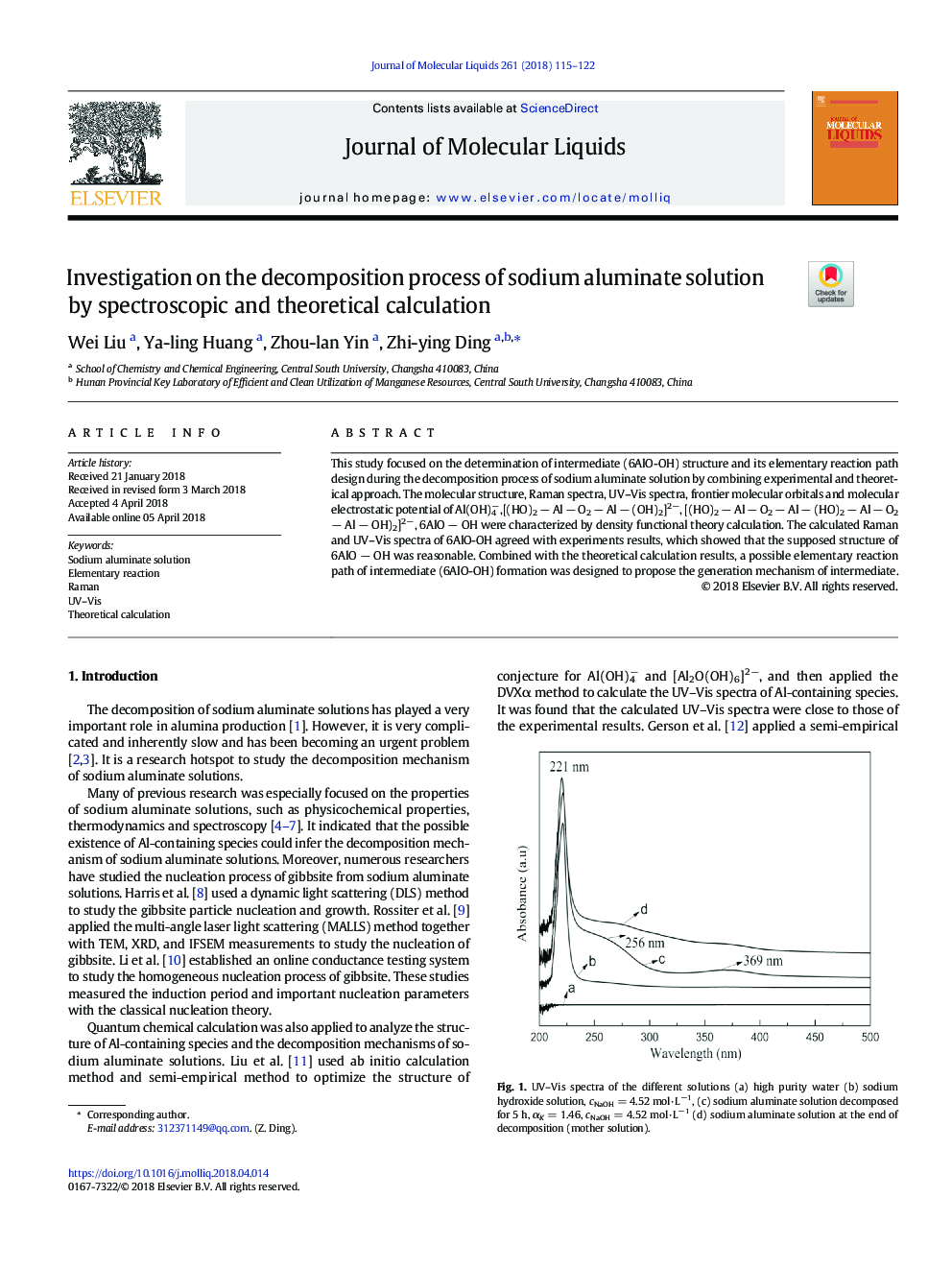
This study focused on the determination of intermediate (6AlO-OH) structure and its elementary reaction path design during the decomposition process of sodium aluminate solution by combining experimental and theoretical approach. The molecular structure, Raman spectra, UV-Vis spectra, frontier molecular orbitals and molecular electrostatic potential of Al(OH)4â,[(HO)2 â Al â O2 â Al â (OH)2]2â, [(HO)2 â Al â O2 â Al â (HO)2 â Al â O2 â Al â OH)2]2â, 6AlO â OH were characterized by density functional theory calculation. The calculated Raman and UV-Vis spectra of 6AlO-OH agreed with experiments results, which showed that the supposed structure of 6AlO â OH was reasonable. Combined with the theoretical calculation results, a possible elementary reaction path of intermediate (6AlO-OH) formation was designed to propose the generation mechanism of intermediate.
Related Topics
Physical Sciences and Engineering
Chemistry
Physical and Theoretical Chemistry
Authors
Wei Liu, Ya-ling Huang, Zhou-lan Yin, Zhi-ying Ding,