| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 9610307 | Catalysis Today | 2005 | 6 Pages |
Abstract
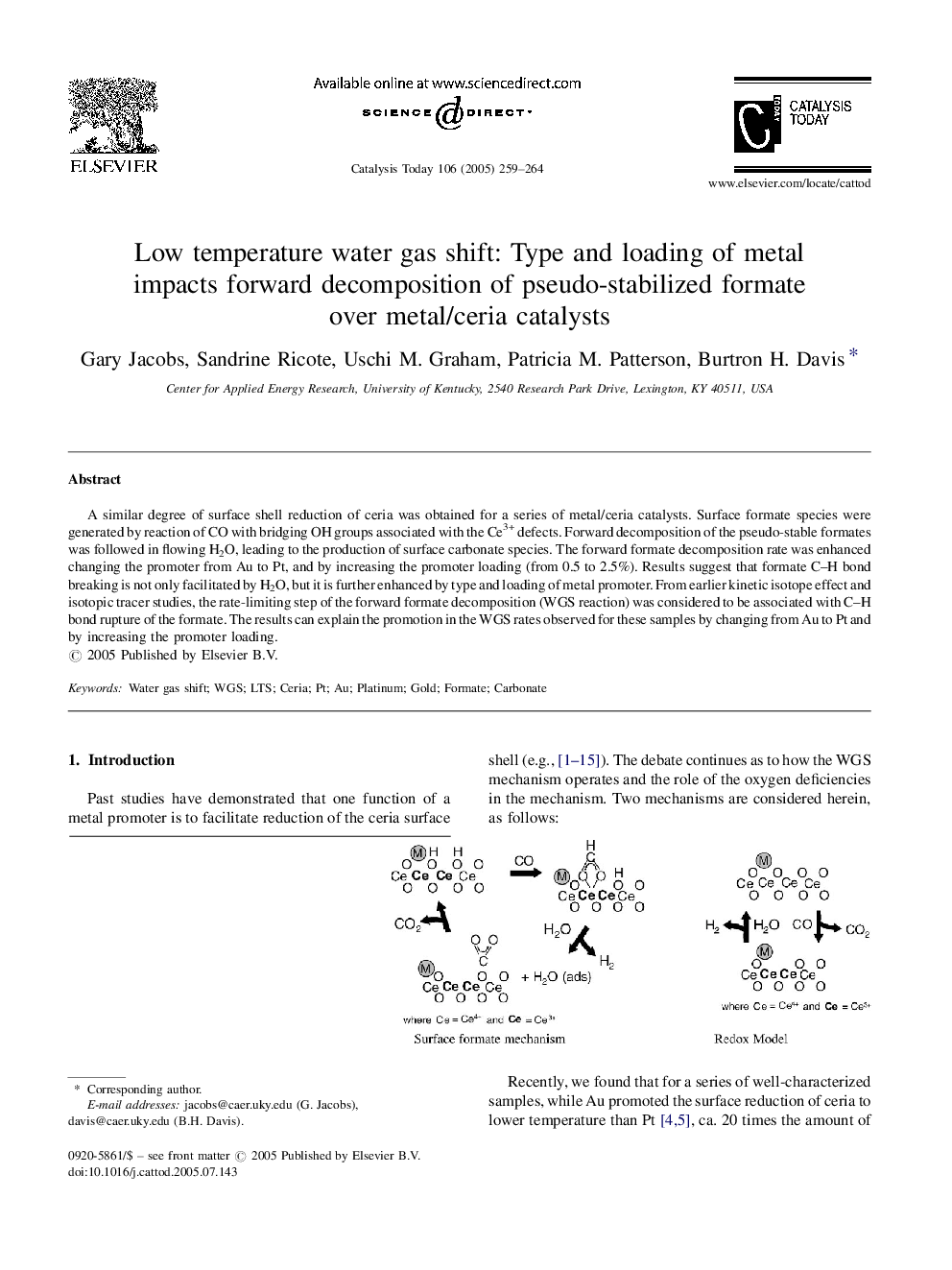
A similar degree of surface shell reduction of ceria was obtained for a series of metal/ceria catalysts. Surface formate species were generated by reaction of CO with bridging OH groups associated with the Ce3+ defects. Forward decomposition of the pseudo-stable formates was followed in flowing H2O, leading to the production of surface carbonate species. The forward formate decomposition rate was enhanced changing the promoter from Au to Pt, and by increasing the promoter loading (from 0.5 to 2.5%). Results suggest that formate CH bond breaking is not only facilitated by H2O, but it is further enhanced by type and loading of metal promoter. From earlier kinetic isotope effect and isotopic tracer studies, the rate-limiting step of the forward formate decomposition (WGS reaction) was considered to be associated with CH bond rupture of the formate. The results can explain the promotion in the WGS rates observed for these samples by changing from Au to Pt and by increasing the promoter loading.
Related Topics
Physical Sciences and Engineering
Chemical Engineering
Catalysis
Authors
Gary Jacobs, Sandrine Ricote, Uschi M. Graham, Patricia M. Patterson, Burtron H. Davis,