| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 10755193 | Biochemical and Biophysical Research Communications | 2014 | 5 Pages |
Abstract
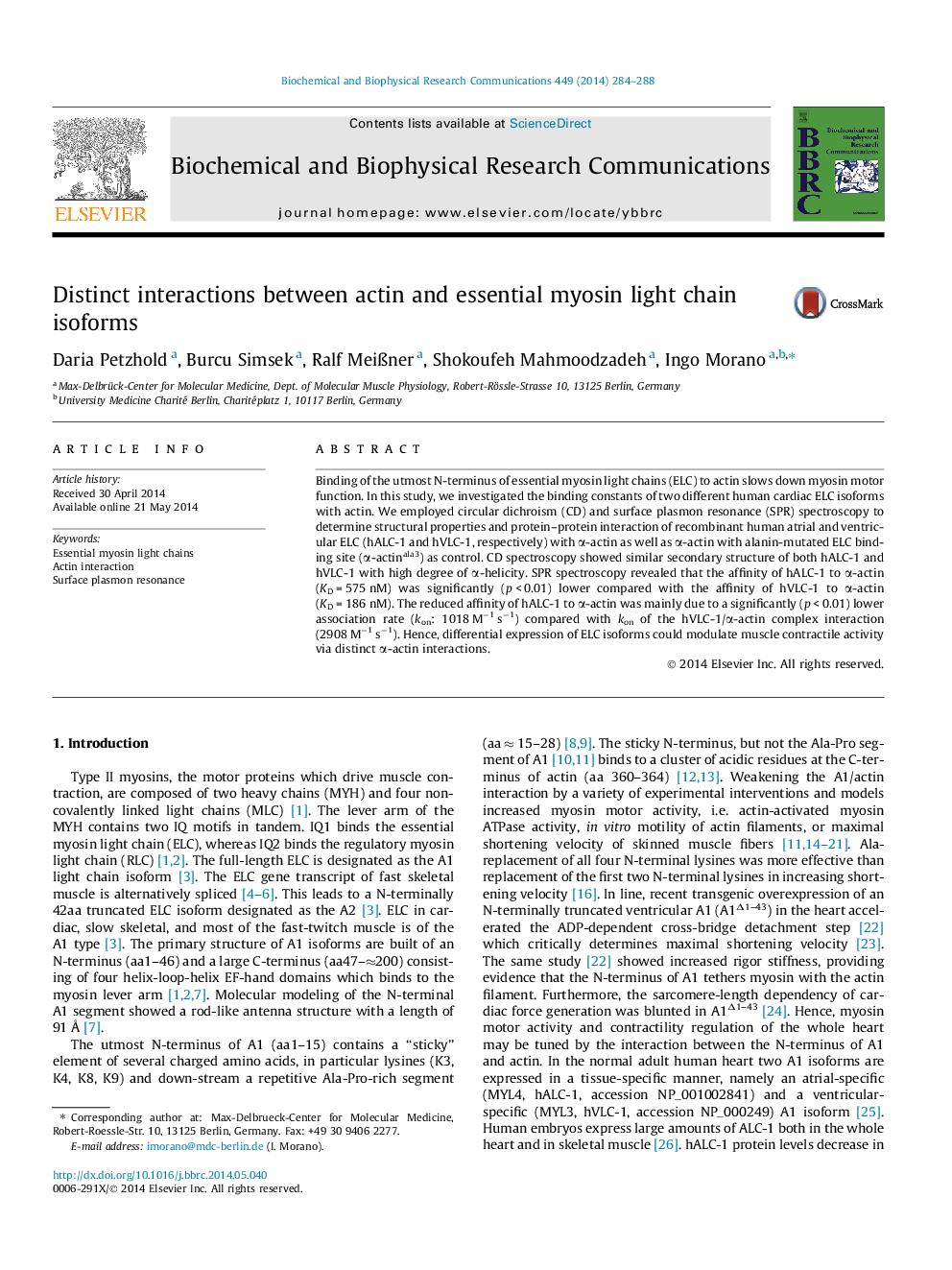
Scheme of the interaction between the utmost lysine-rich (K3, K4, K8, K9) domain of the N-terminus (red) of essential myosin light chain (A1) and a cluster of negatively charged amino acids (E360, D362, E363) of actin. In this paper, we characterized the dissociation constants (KD) of the atrial-specific and ventricular-specific A1 (hALC-1 and hVLC-1, respectively). KD of the hALC-1/actin complex was 575 nM, i.e. significantly (p < 0.01) 3fold higher than KD of the hVLC-1/actin complex (189 nM. Binding of A1 to actin slows down cardiac shortening velocity. Partial replacement of hVLC-1 by hALC-1, e.g. in the hypertrophied human ventricle weakens the inhibitory A1/actin interaction, thus increasing shortening velocity. hALC-1 expression, therefore represents an auto-regulatory mechanism to adapt the human heart to an increased work demand. 3D-structures were obtained from PDB1ATN (G-actin) and the coordinates from Ref. [7] (A1).
Keywords
Related Topics
Life Sciences
Biochemistry, Genetics and Molecular Biology
Biochemistry
Authors
Daria Petzhold, Burcu Simsek, Ralf MeiÃner, Shokoufeh Mahmoodzadeh, Ingo Morano,