| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1162968 | Analytica Chimica Acta | 2016 | 10 Pages |
•A ternary TiO2 nanotube–polyaniline–gold nanoparticle composite was developed.•New synthetic route for ternary composite with a polyaniline molecular wire between TiO2 nanotubes and gold nanoparticles.•An electrochemical biosensor with ternary composite as a scaffold.•Ternary composite facilitated improved analytical performance of electrochemical biosensor.
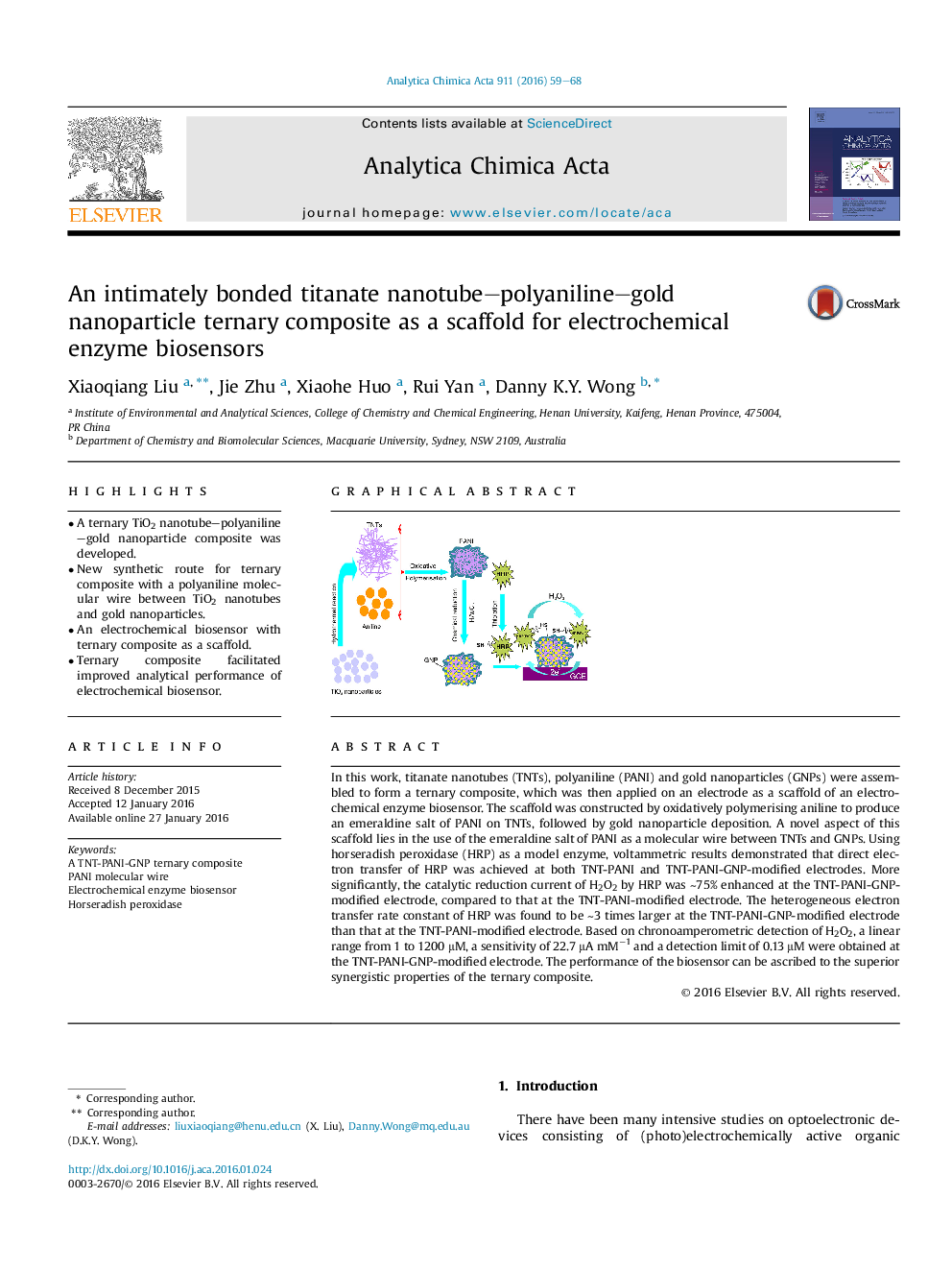
In this work, titanate nanotubes (TNTs), polyaniline (PANI) and gold nanoparticles (GNPs) were assembled to form a ternary composite, which was then applied on an electrode as a scaffold of an electrochemical enzyme biosensor. The scaffold was constructed by oxidatively polymerising aniline to produce an emeraldine salt of PANI on TNTs, followed by gold nanoparticle deposition. A novel aspect of this scaffold lies in the use of the emeraldine salt of PANI as a molecular wire between TNTs and GNPs. Using horseradish peroxidase (HRP) as a model enzyme, voltammetric results demonstrated that direct electron transfer of HRP was achieved at both TNT-PANI and TNT-PANI-GNP-modified electrodes. More significantly, the catalytic reduction current of H2O2 by HRP was ∼75% enhanced at the TNT-PANI-GNP-modified electrode, compared to that at the TNT-PANI-modified electrode. The heterogeneous electron transfer rate constant of HRP was found to be ∼3 times larger at the TNT-PANI-GNP-modified electrode than that at the TNT-PANI-modified electrode. Based on chronoamperometric detection of H2O2, a linear range from 1 to 1200 μM, a sensitivity of 22.7 μA mM−1 and a detection limit of 0.13 μM were obtained at the TNT-PANI-GNP-modified electrode. The performance of the biosensor can be ascribed to the superior synergistic properties of the ternary composite.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide