| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1162979 | Analytica Chimica Acta | 2016 | 7 Pages |
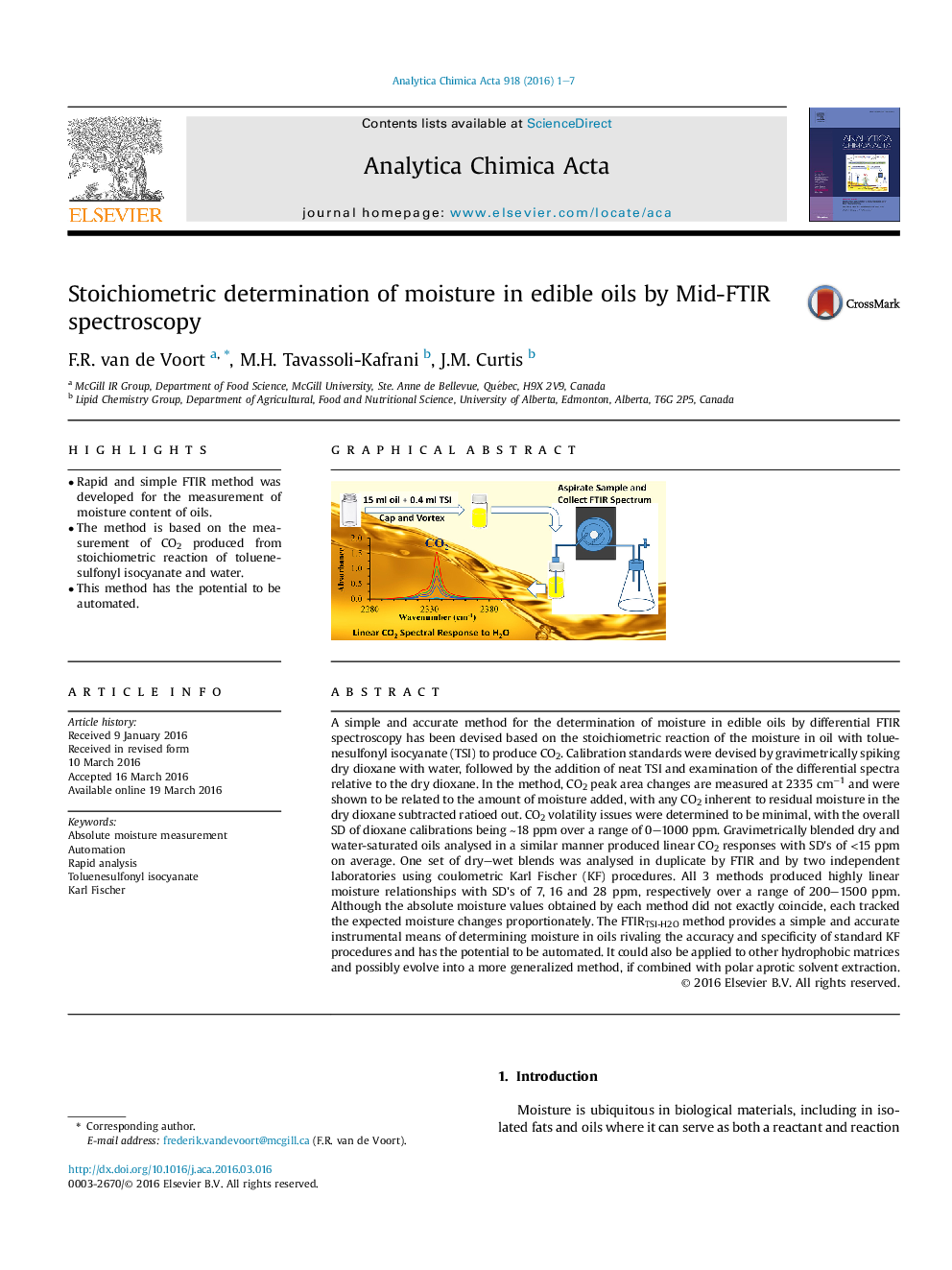
•Rapid and simple FTIR method was developed for the measurement of moisture content of oils.•The method is based on the measurement of CO2 produced from stoichiometric reaction of toluenesulfonyl isocyanate and water.•This method has the potential to be automated.
A simple and accurate method for the determination of moisture in edible oils by differential FTIR spectroscopy has been devised based on the stoichiometric reaction of the moisture in oil with toluenesulfonyl isocyanate (TSI) to produce CO2. Calibration standards were devised by gravimetrically spiking dry dioxane with water, followed by the addition of neat TSI and examination of the differential spectra relative to the dry dioxane. In the method, CO2 peak area changes are measured at 2335 cm−1 and were shown to be related to the amount of moisture added, with any CO2 inherent to residual moisture in the dry dioxane subtracted ratioed out. CO2 volatility issues were determined to be minimal, with the overall SD of dioxane calibrations being ∼18 ppm over a range of 0–1000 ppm. Gravimetrically blended dry and water-saturated oils analysed in a similar manner produced linear CO2 responses with SD's of <15 ppm on average. One set of dry–wet blends was analysed in duplicate by FTIR and by two independent laboratories using coulometric Karl Fischer (KF) procedures. All 3 methods produced highly linear moisture relationships with SD's of 7, 16 and 28 ppm, respectively over a range of 200–1500 ppm. Although the absolute moisture values obtained by each method did not exactly coincide, each tracked the expected moisture changes proportionately. The FTIRTSI-H2O method provides a simple and accurate instrumental means of determining moisture in oils rivaling the accuracy and specificity of standard KF procedures and has the potential to be automated. It could also be applied to other hydrophobic matrices and possibly evolve into a more generalized method, if combined with polar aprotic solvent extraction.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide