| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1163235 | Analytica Chimica Acta | 2015 | 7 Pages |
•E. coli surface-dispalyed Gldh exhibiting excellent enzyme activity and stability.•Sensitive amperometric biosensor for glutamate using Gldh-bacteria and MWNTs.•The glutamate biosensor exhibited high specificity and stability.
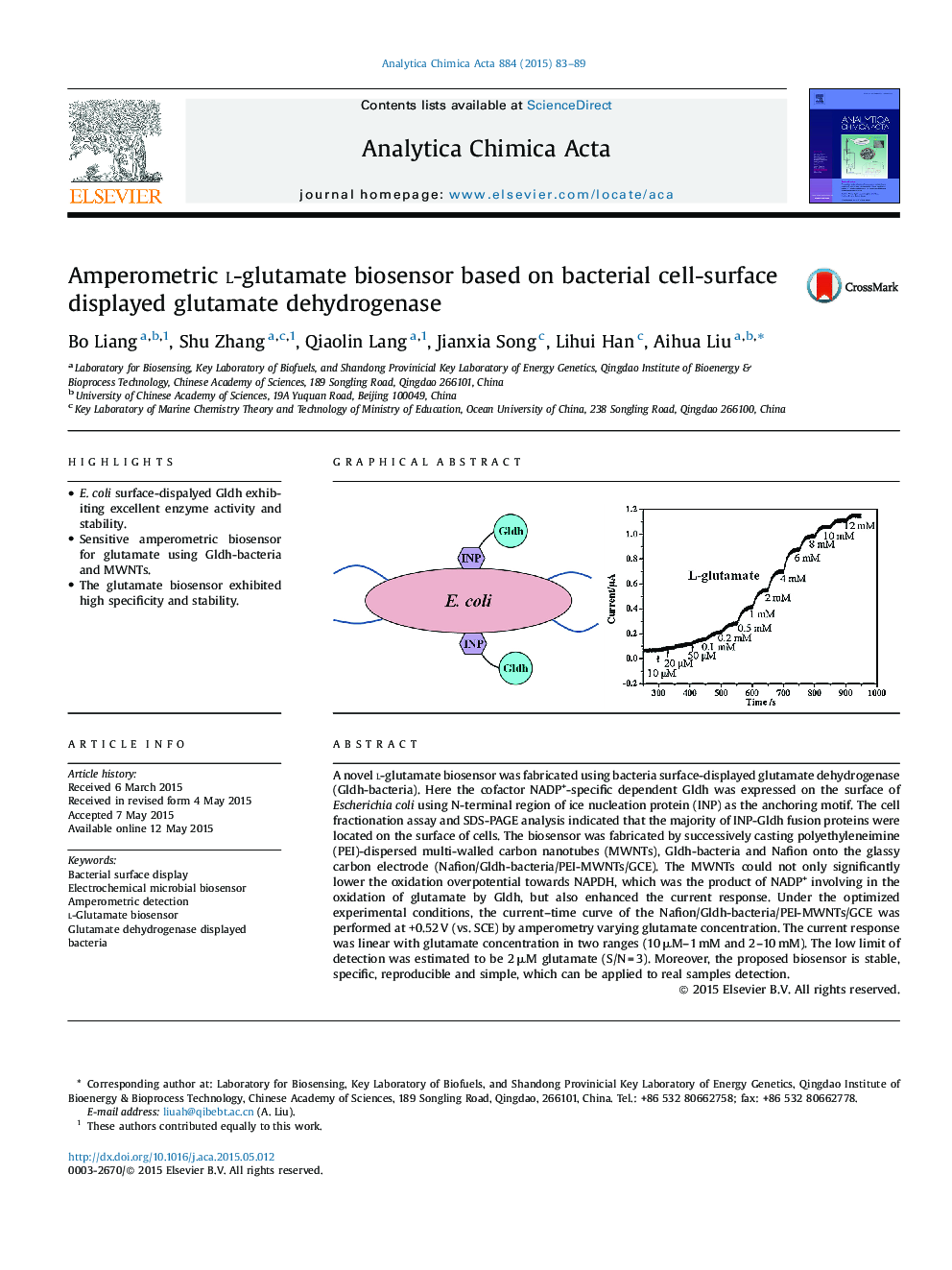
A novel l-glutamate biosensor was fabricated using bacteria surface-displayed glutamate dehydrogenase (Gldh-bacteria). Here the cofactor NADP+-specific dependent Gldh was expressed on the surface of Escherichia coli using N-terminal region of ice nucleation protein (INP) as the anchoring motif. The cell fractionation assay and SDS-PAGE analysis indicated that the majority of INP-Gldh fusion proteins were located on the surface of cells. The biosensor was fabricated by successively casting polyethyleneimine (PEI)-dispersed multi-walled carbon nanotubes (MWNTs), Gldh-bacteria and Nafion onto the glassy carbon electrode (Nafion/Gldh-bacteria/PEI-MWNTs/GCE). The MWNTs could not only significantly lower the oxidation overpotential towards NAPDH, which was the product of NADP+ involving in the oxidation of glutamate by Gldh, but also enhanced the current response. Under the optimized experimental conditions, the current–time curve of the Nafion/Gldh-bacteria/PEI-MWNTs/GCE was performed at +0.52 V (vs. SCE) by amperometry varying glutamate concentration. The current response was linear with glutamate concentration in two ranges (10 μM–1 mM and 2–10 mM). The low limit of detection was estimated to be 2 μM glutamate (S/N = 3). Moreover, the proposed biosensor is stable, specific, reproducible and simple, which can be applied to real samples detection.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide