| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1164277 | Analytica Chimica Acta | 2015 | 9 Pages |
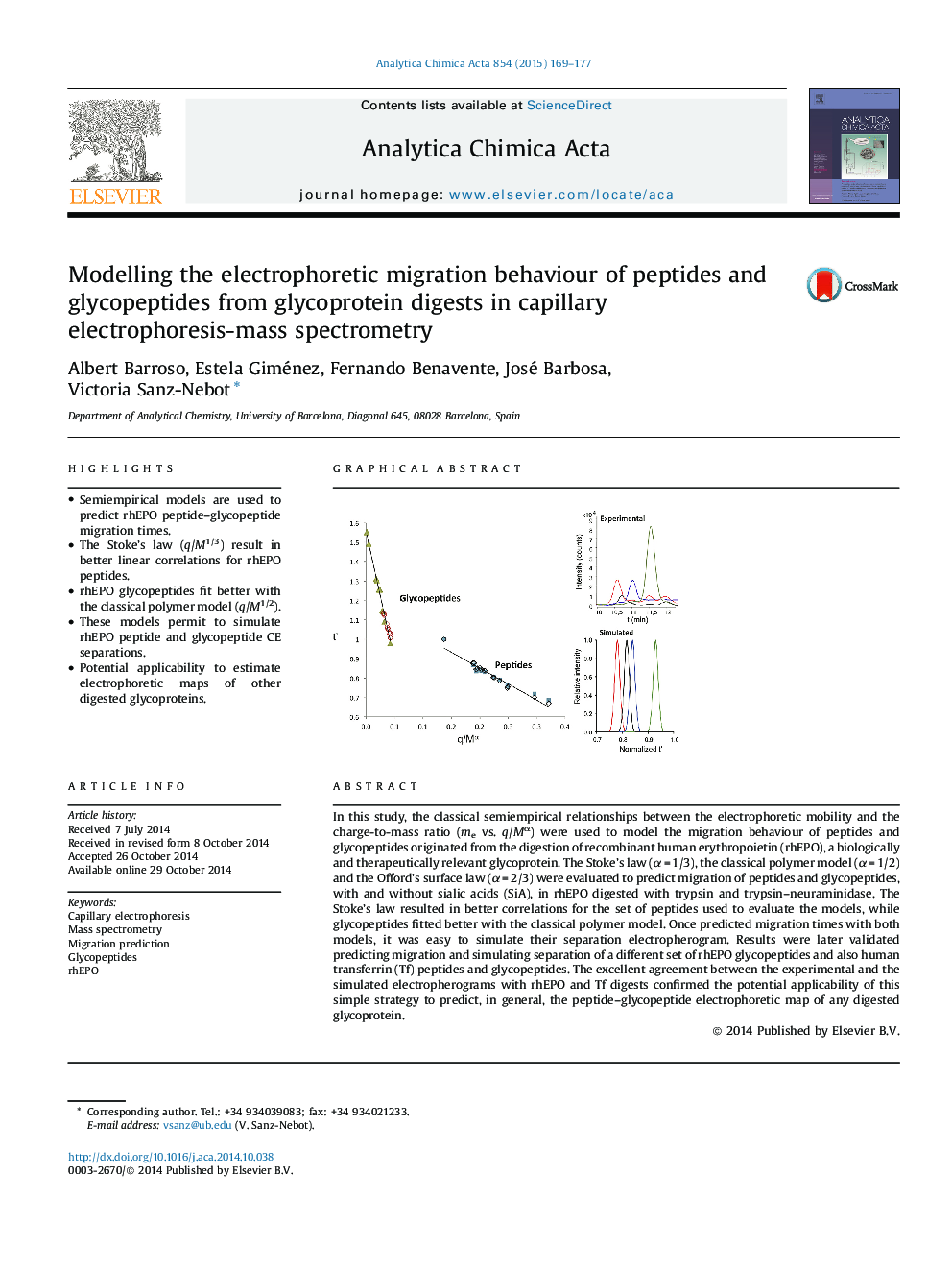
•Semiempirical models are used to predict rhEPO peptide–glycopeptide migration times.•The Stoke’s law (q/M1/3) result in better linear correlations for rhEPO peptides.•rhEPO glycopeptides fit better with the classical polymer model (q/M1/2).•These models permit to simulate rhEPO peptide and glycopeptide CE separations.•Potential applicability to estimate electrophoretic maps of other digested glycoproteins.
In this study, the classical semiempirical relationships between the electrophoretic mobility and the charge-to-mass ratio (me vs. q/Mα) were used to model the migration behaviour of peptides and glycopeptides originated from the digestion of recombinant human erythropoietin (rhEPO), a biologically and therapeutically relevant glycoprotein. The Stoke’s law (α = 1/3), the classical polymer model (α = 1/2) and the Offord’s surface law (α = 2/3) were evaluated to predict migration of peptides and glycopeptides, with and without sialic acids (SiA), in rhEPO digested with trypsin and trypsin–neuraminidase. The Stoke’s law resulted in better correlations for the set of peptides used to evaluate the models, while glycopeptides fitted better with the classical polymer model. Once predicted migration times with both models, it was easy to simulate their separation electropherogram. Results were later validated predicting migration and simulating separation of a different set of rhEPO glycopeptides and also human transferrin (Tf) peptides and glycopeptides. The excellent agreement between the experimental and the simulated electropherograms with rhEPO and Tf digests confirmed the potential applicability of this simple strategy to predict, in general, the peptide–glycopeptide electrophoretic map of any digested glycoprotein.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide