| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1166203 | Analytica Chimica Acta | 2012 | 10 Pages |
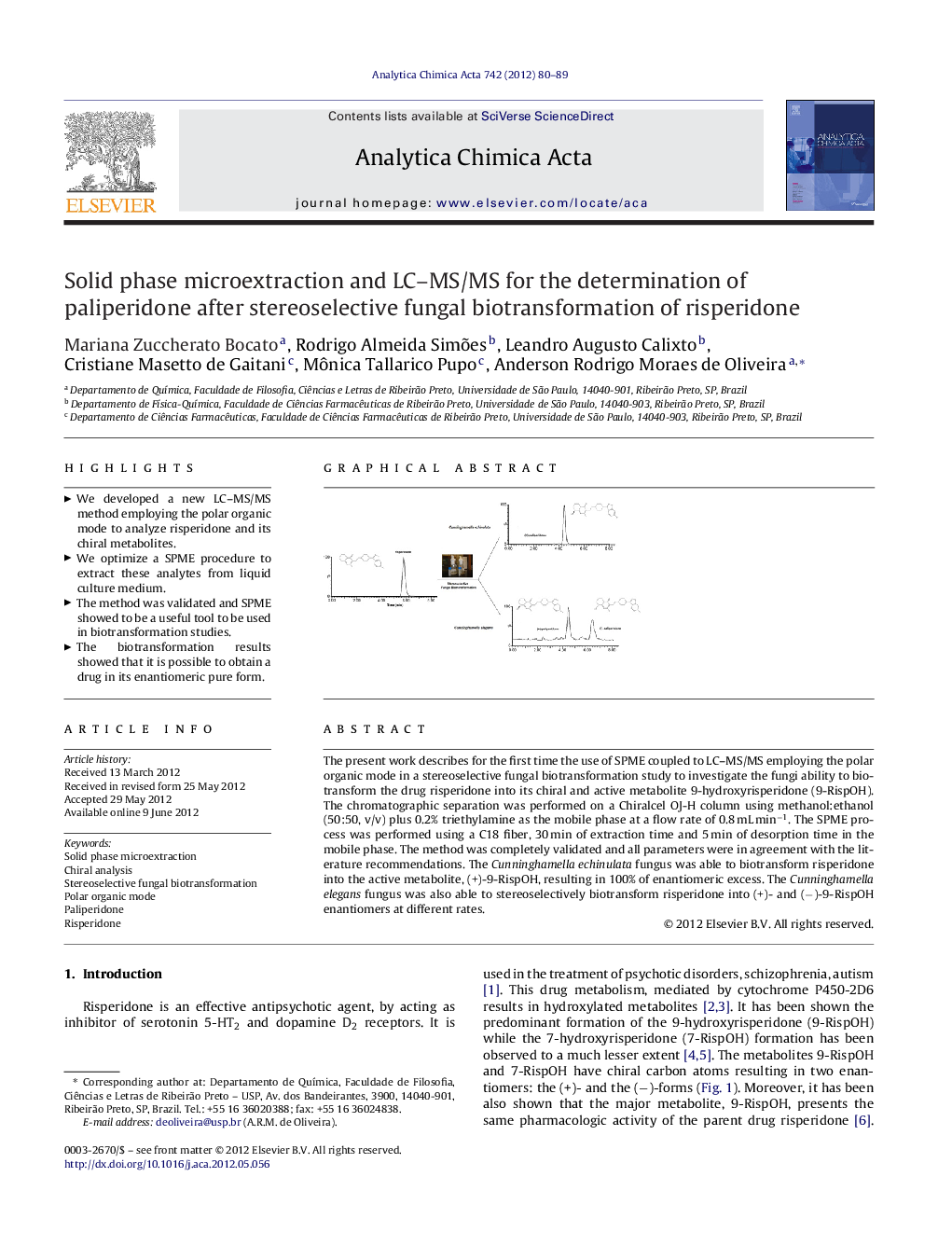
The present work describes for the first time the use of SPME coupled to LC–MS/MS employing the polar organic mode in a stereoselective fungal biotransformation study to investigate the fungi ability to biotransform the drug risperidone into its chiral and active metabolite 9-hydroxyrisperidone (9-RispOH). The chromatographic separation was performed on a Chiralcel OJ-H column using methanol:ethanol (50:50, v/v) plus 0.2% triethylamine as the mobile phase at a flow rate of 0.8 mL min−1. The SPME process was performed using a C18 fiber, 30 min of extraction time and 5 min of desorption time in the mobile phase. The method was completely validated and all parameters were in agreement with the literature recommendations. The Cunninghamella echinulata fungus was able to biotransform risperidone into the active metabolite, (+)-9-RispOH, resulting in 100% of enantiomeric excess. The Cunninghamella elegans fungus was also able to stereoselectively biotransform risperidone into (+)- and (−)-9-RispOH enantiomers at different rates.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► We developed a new LC–MS/MS method employing the polar organic mode to analyze risperidone and its chiral metabolites. ► We optimize a SPME procedure to extract these analytes from liquid culture medium. ► The method was validated and SPME showed to be a useful tool to be used in biotransformation studies. ► The biotransformation results showed that it is possible to obtain a drug in its enantiomeric pure form.