| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229479 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 10 Pages |
•The complexes are characterized by different spectroscopic techniques.•The Schiff base ligand exhibited efficient corrosion inhibitors.•The ligand and its complexes exhibited intraligand (π–π∗) fluorescence and can potentially serve as photoactive materials.•The thermal analyses confirmed high stability for all complexes.
A series of eight metal Schiff base complexes were synthesized by the thermal reaction of Cu(II), Ni(II), Fe(III), Co(II), Zn(II), Hg(II), La(III) or Sm(III) with a Schiff base “L” produced by the condensation of furfuraldehyde and 1,2-diaminobenzene. These compounds were characterized by elemental analysis, UV–Vis, FT-IR, molar conductance, mass spectrometry, thermal and fluorescence studies. The studies suggested the coordination of the ligand L to metal through azomethine imine nitrogen and furan oxygen atoms of Schiff base moiety. Thermogravimetric (TG/DTG) analyses data were studied and indicated high stability for all complexes and suggested the presence of lattice and/or coordinated water molecules in the complexes. Coats–Redfern method has been used to calculate the kinetic and thermodynamic parameters of the metal complexes. The spectral and thermal analysis reveal that all complexes have octahedral geometry except Cu(II) and Ni(II) complexes which can attain a square planner arrangements. The ligand and its complexes exhibited intraligand (π–π∗) fluorescence and can potentially serve as photoactive materials. Both the ligand and its complexes have been screened for antibacterial activities.
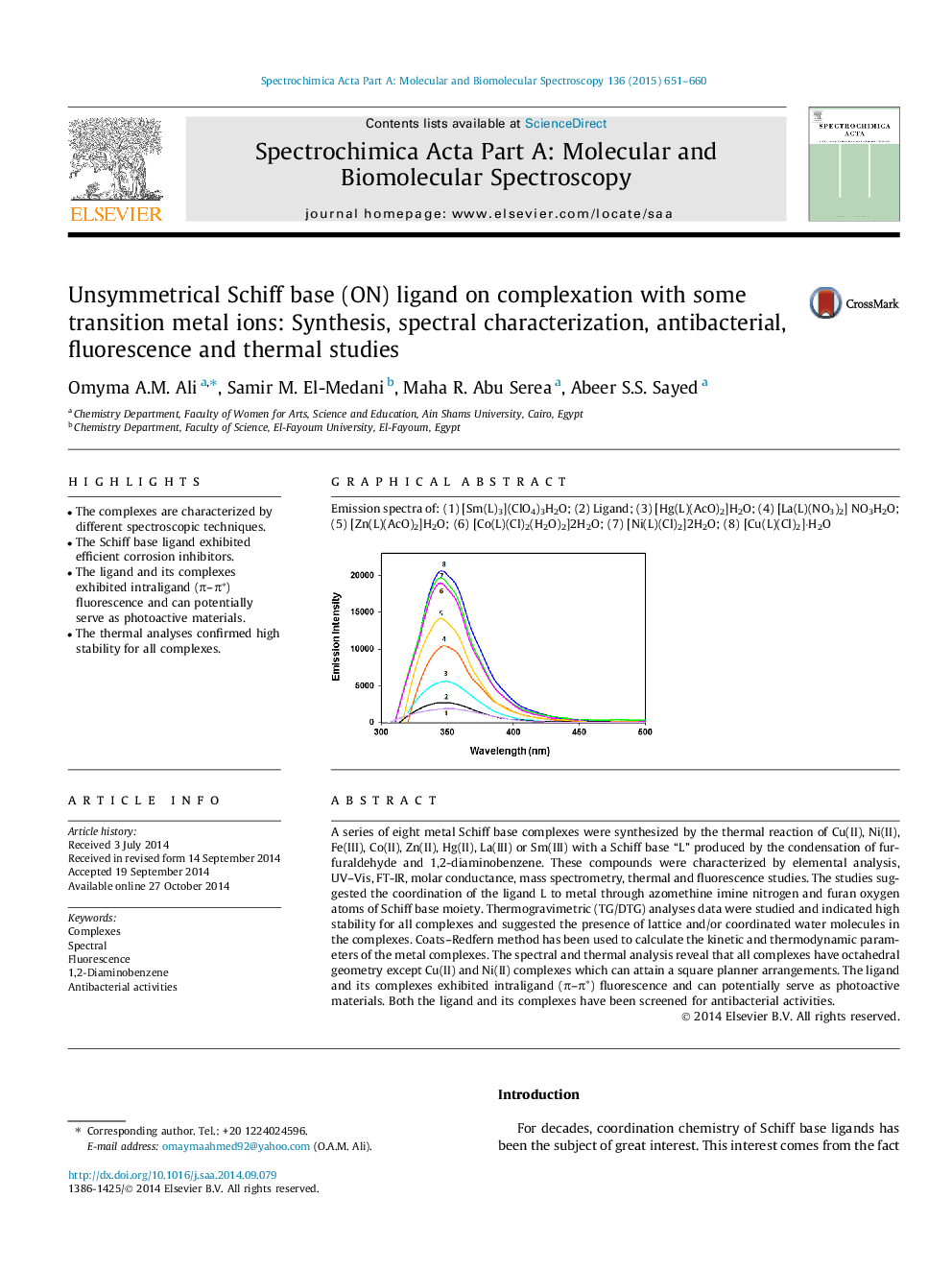
Graphical abstractEmission spectra of: (1) [Sm(L)3](ClO4)3H2O; (2) Ligand; (3) [Hg(L)(AcO)2]H2O; (4) [La(L)(NO3)2] NO3H2O; (5) [Zn(L)(AcO)2]H2O; (6) [Co(L)(Cl)2(H2O)2]2H2O; (7) [Ni(L)(Cl)2]2H2O; (8) [Cu(L)(Cl)2]·H2OFigure optionsDownload full-size imageDownload as PowerPoint slide