| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229753 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2016 | 8 Pages |
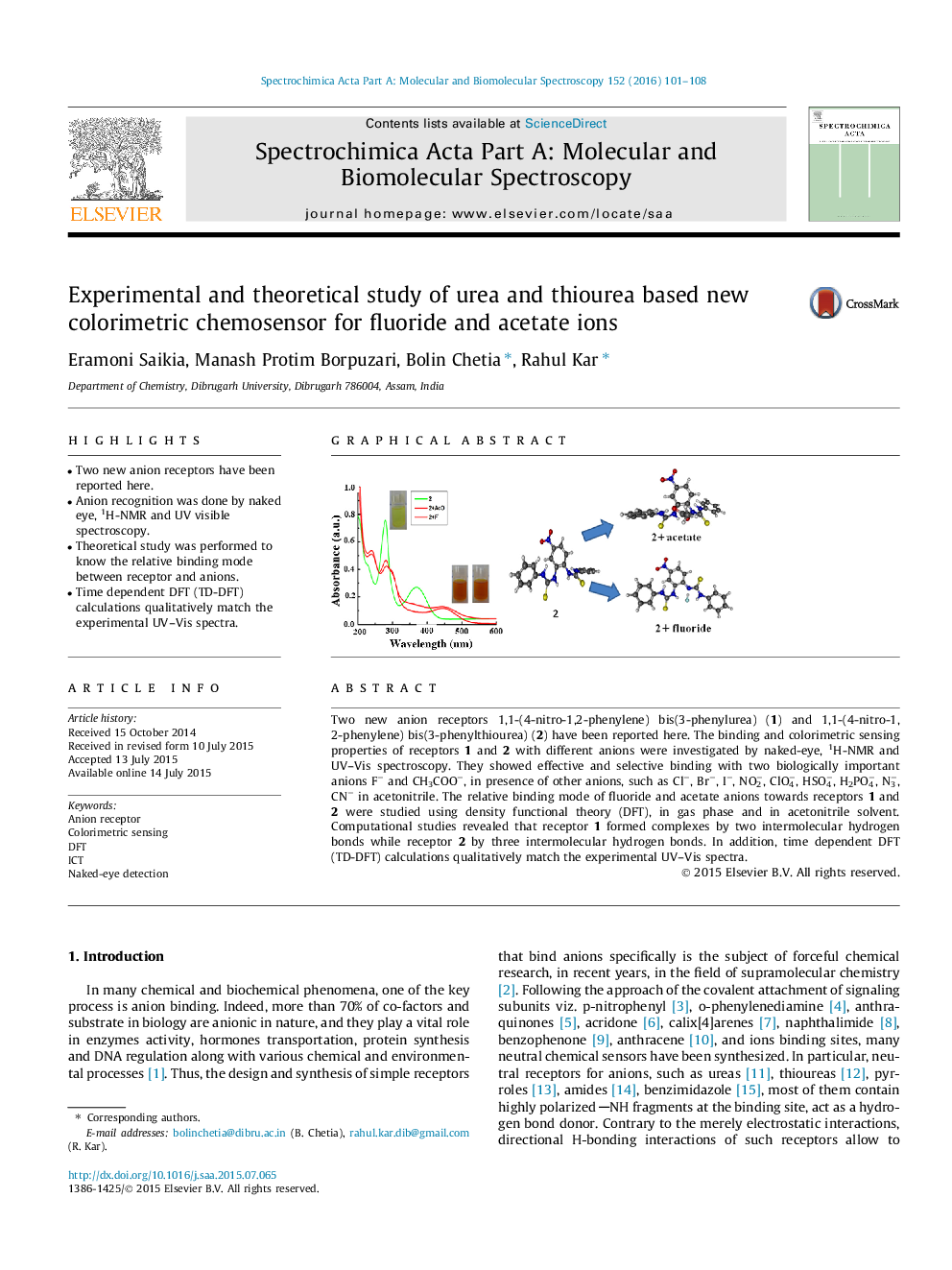
•Two new anion receptors have been reported here.•Anion recognition was done by naked eye, 1H-NMR and UV visible spectroscopy.•Theoretical study was performed to know the relative binding mode between receptor and anions.•Time dependent DFT (TD-DFT) calculations qualitatively match the experimental UV–Vis spectra.
Two new anion receptors 1,1-(4-nitro-1,2-phenylene) bis(3-phenylurea) (1) and 1,1-(4-nitro-1,2-phenylene) bis(3-phenylthiourea) (2) have been reported here. The binding and colorimetric sensing properties of receptors 1 and 2 with different anions were investigated by naked-eye, 1H-NMR and UV–Vis spectroscopy. They showed effective and selective binding with two biologically important anions F− and CH3COO−, in presence of other anions, such as Cl−, Br−, I−, NO2−, ClO4−, HSO4−, H2PO4−, N3−, CN− in acetonitrile. The relative binding mode of fluoride and acetate anions towards receptors 1 and 2 were studied using density functional theory (DFT), in gas phase and in acetonitrile solvent. Computational studies revealed that receptor 1 formed complexes by two intermolecular hydrogen bonds while receptor 2 by three intermolecular hydrogen bonds. In addition, time dependent DFT (TD-DFT) calculations qualitatively match the experimental UV–Vis spectra.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide