| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1283407 | Journal of Power Sources | 2016 | 9 Pages |
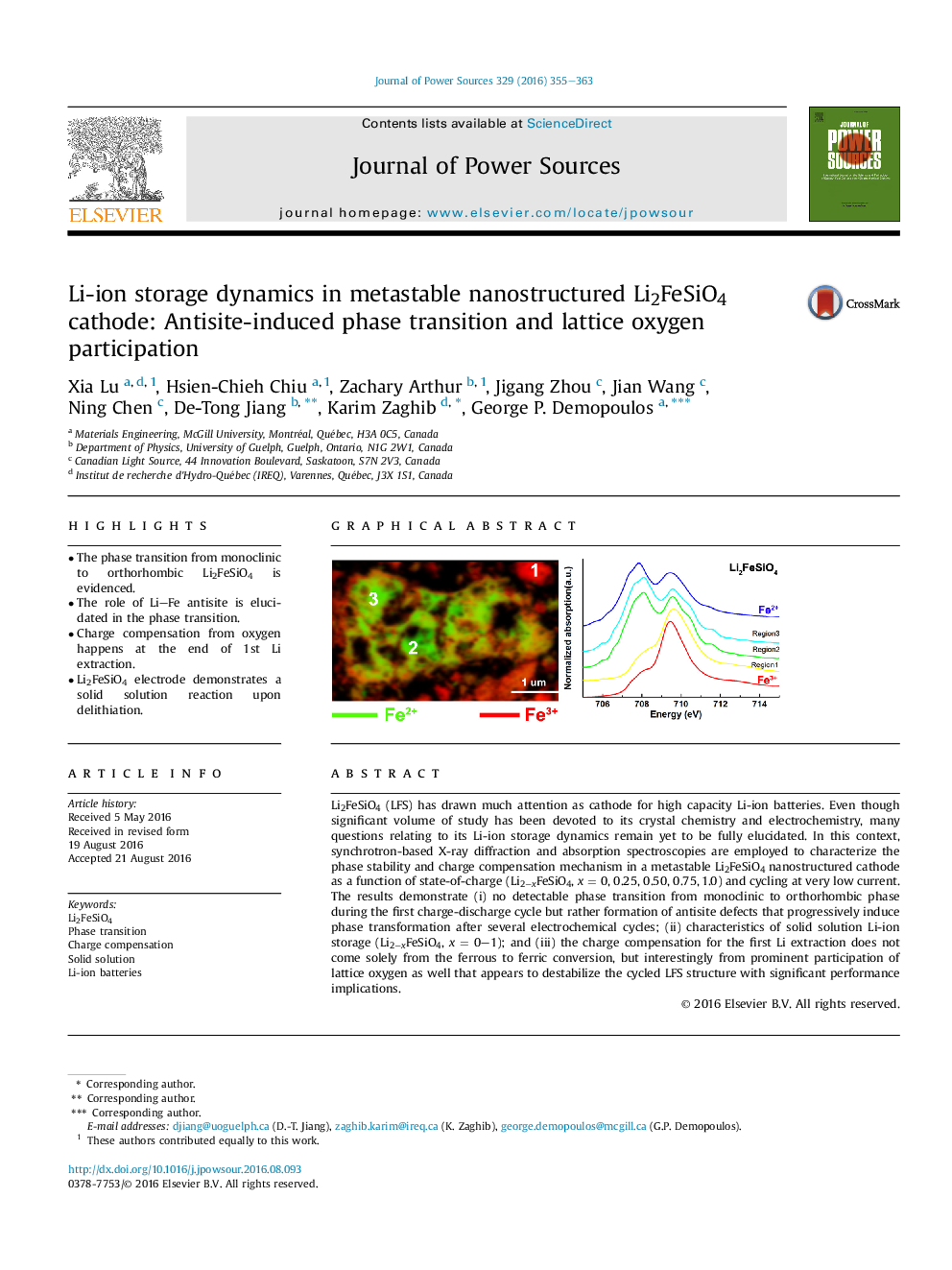
•The phase transition from monoclinic to orthorhombic Li2FeSiO4 is evidenced.•The role of LiFe antisite is elucidated in the phase transition.•Charge compensation from oxygen happens at the end of 1st Li extraction.•Li2FeSiO4 electrode demonstrates a solid solution reaction upon delithiation.
Li2FeSiO4 (LFS) has drawn much attention as cathode for high capacity Li-ion batteries. Even though significant volume of study has been devoted to its crystal chemistry and electrochemistry, many questions relating to its Li-ion storage dynamics remain yet to be fully elucidated. In this context, synchrotron-based X-ray diffraction and absorption spectroscopies are employed to characterize the phase stability and charge compensation mechanism in a metastable Li2FeSiO4 nanostructured cathode as a function of state-of-charge (Li2−xFeSiO4, x = 0, 0.25, 0.50, 0.75, 1.0) and cycling at very low current. The results demonstrate (i) no detectable phase transition from monoclinic to orthorhombic phase during the first charge-discharge cycle but rather formation of antisite defects that progressively induce phase transformation after several electrochemical cycles; (ii) characteristics of solid solution Li-ion storage (Li2−xFeSiO4, x = 0–1); and (iii) the charge compensation for the first Li extraction does not come solely from the ferrous to ferric conversion, but interestingly from prominent participation of lattice oxygen as well that appears to destabilize the cycled LFS structure with significant performance implications.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide