| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1283403 | Journal of Power Sources | 2016 | 7 Pages |
•Core-shell structured Fe-HCF@Ni-HCF was synthesized via a facile solution method.•The Fe-HCF@Ni-HCF composite integrates the advantages of both Fe-HCF and Ni-HCF.•Ni-HCF coating improves the electron conductivity and suppresses the side reactions.
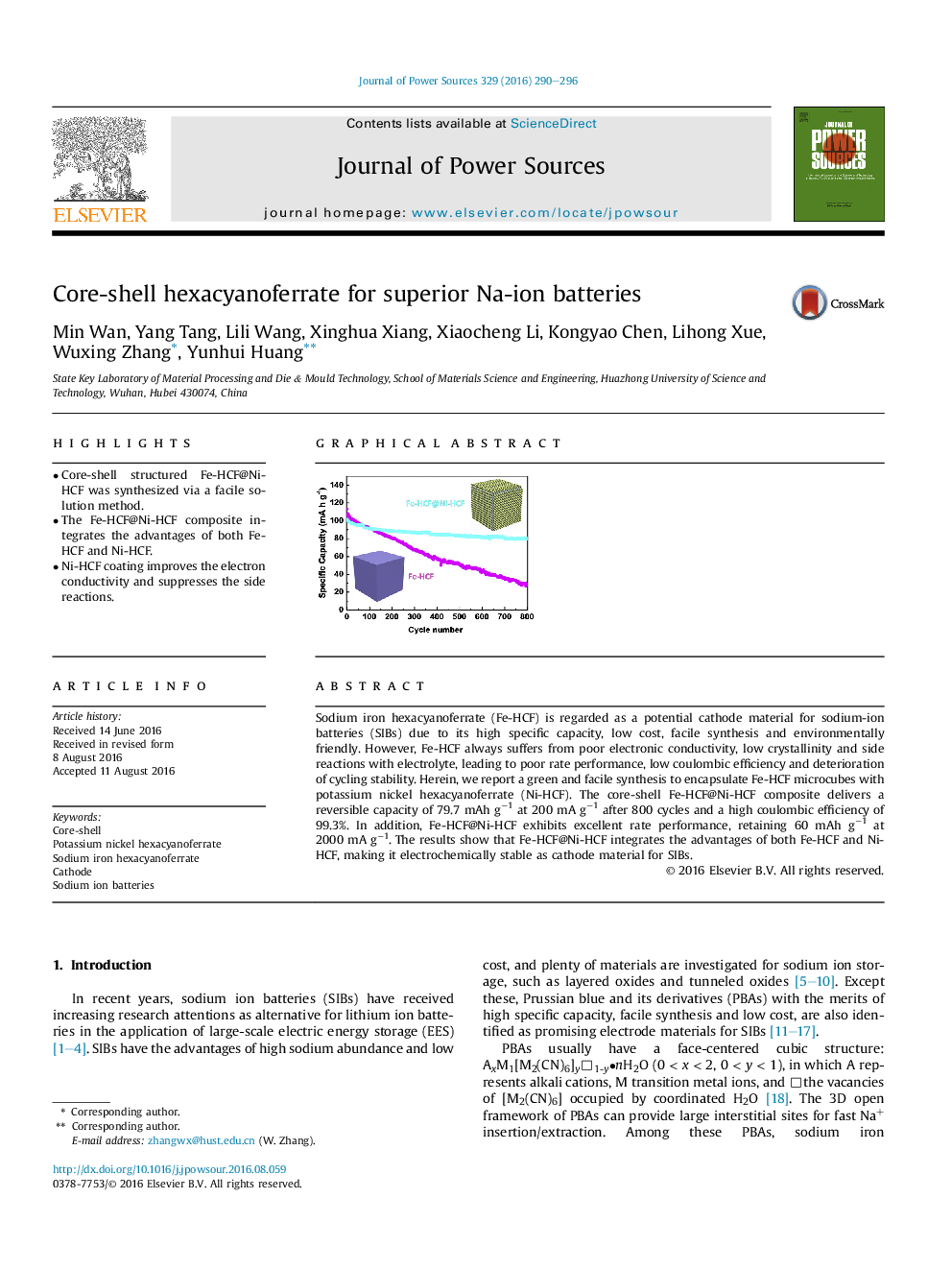
Sodium iron hexacyanoferrate (Fe-HCF) is regarded as a potential cathode material for sodium-ion batteries (SIBs) due to its high specific capacity, low cost, facile synthesis and environmentally friendly. However, Fe-HCF always suffers from poor electronic conductivity, low crystallinity and side reactions with electrolyte, leading to poor rate performance, low coulombic efficiency and deterioration of cycling stability. Herein, we report a green and facile synthesis to encapsulate Fe-HCF microcubes with potassium nickel hexacyanoferrate (Ni-HCF). The core-shell Fe-HCF@Ni-HCF composite delivers a reversible capacity of 79.7 mAh g−1 at 200 mA g−1 after 800 cycles and a high coulombic efficiency of 99.3%. In addition, Fe-HCF@Ni-HCF exhibits excellent rate performance, retaining 60 mAh g−1 at 2000 mA g−1. The results show that Fe-HCF@Ni-HCF integrates the advantages of both Fe-HCF and Ni-HCF, making it electrochemically stable as cathode material for SIBs.
Graphical abstractFe-HCF@Ni-HCF composite exhibits superior cycling stability and improved coulombic efficiency due to Ni-HCF as a protective layer to prevent the side reactions.Figure optionsDownload full-size imageDownload as PowerPoint slide