| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1332655 | Journal of Solid State Chemistry | 2012 | 5 Pages |
The formation enthalpies and heat capacities of orthorhombic rare earth titanates, RE2TiO5 (RE=La, Nd and Gd), have been studied by high temperature differential scanning calorimetry (300–1473 K) and oxide-melt solution calorimetry. The RE2TiO5 samples are stable in enthalpy with respect to their oxides and the pyrochlore RE2Ti2O7 phase. The general trend that has been demonstrated in other RE-ternary systems; decreasing thermodynamic stability with decreasing RA/RB was found to be valid for the RE2TiO5, and their enthalpies of formation from oxides become more negative with increasing RE3+ ionic radius.
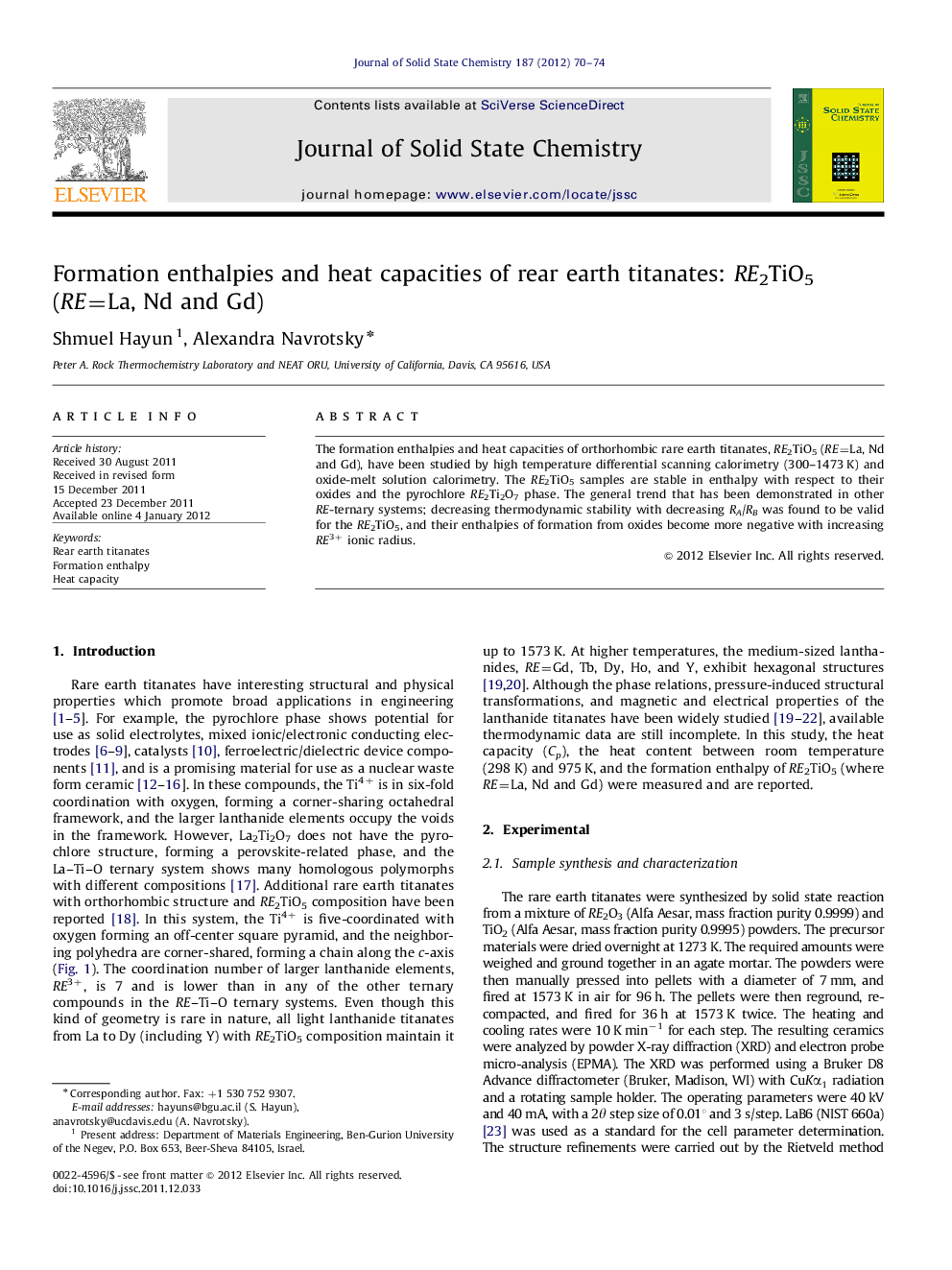
Graphical abstractNormalized enthalpy of formation for one RE3+ cation from the oxides for several RE ternary oxide systems vs. the cation radius ratio RA/RB (A=RE, B=Ti, Zr, P). All the RE ternary oxide systems are stable relative to constituent oxides, with increasing stability as RA/RB increases. The Roman numerals above the cations represent the coordination number.Figure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Formation enthalpies and heat capacities of RE2TiO5 (RE=La, Nd and Gd) were determined. ► Enthalpies of formation of RE2TiO5 from oxides become more negative with increasing RE3+ ionic radius. ► RE2TiO5 phases are stable in enthalpy with respect to their oxides and the pyrochlore RE2Ti2O7 phases. ► Thermodynamic stability of orthorhombic RE2TiO5 decrease with increasing RB to RA ratio.