| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1401998 | Journal of Molecular Structure | 2015 | 6 Pages |
•One novel 2D copper(II) coordination polymer was synthesized and characterized.•A planar water hexamer was trapped in the interstitial void of the coordination polymer.•The formation of water hexamer is reversible by straightforward processes of dehydration/rehydration.
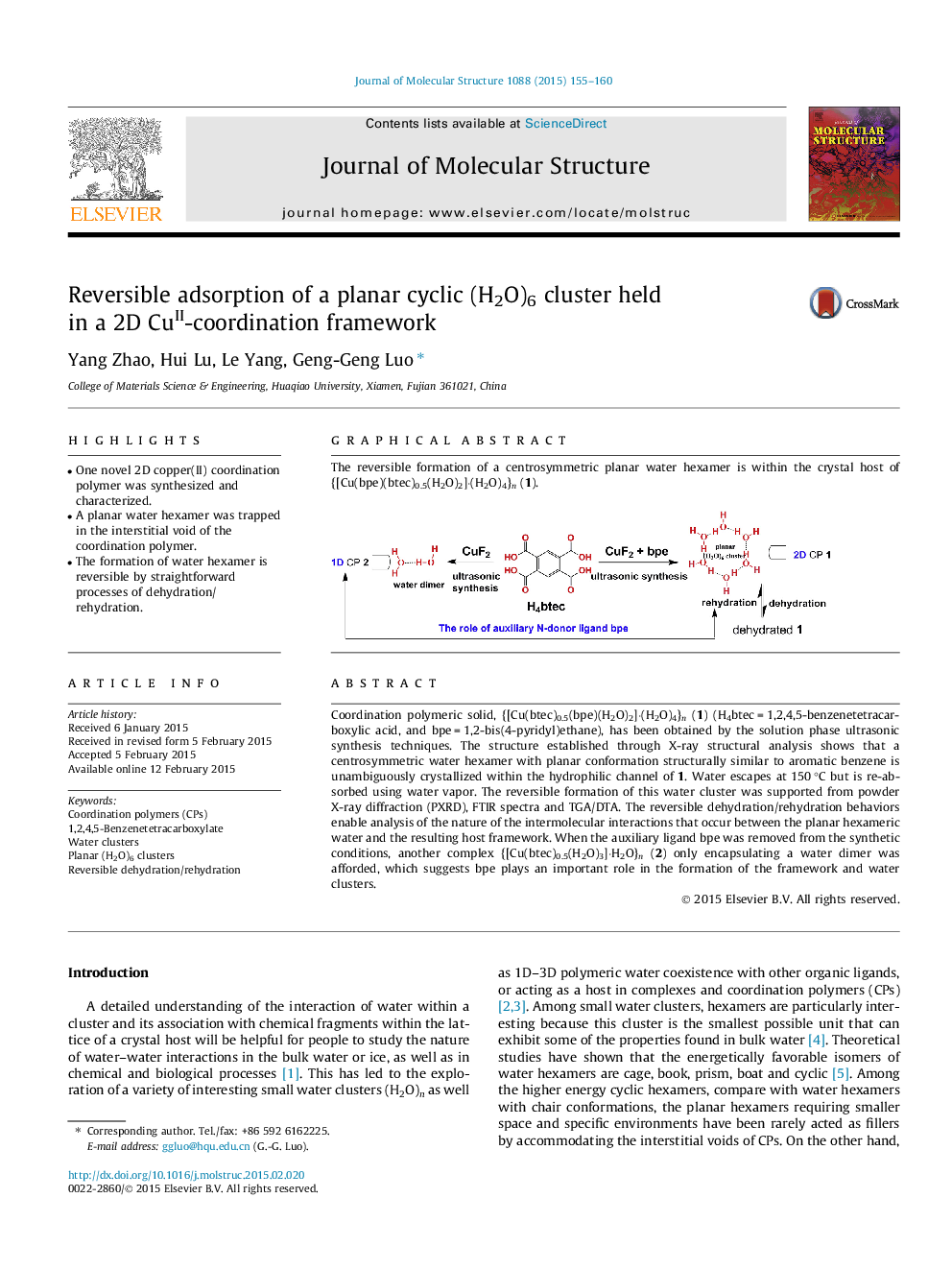
Coordination polymeric solid, {[Cu(btec)0.5(bpe)(H2O)2]⋅(H2O)4}n (1) (H4btec = 1,2,4,5-benzenetetracarboxylic acid, and bpe = 1,2-bis(4-pyridyl)ethane), has been obtained by the solution phase ultrasonic synthesis techniques. The structure established through X-ray structural analysis shows that a centrosymmetric water hexamer with planar conformation structurally similar to aromatic benzene is unambiguously crystallized within the hydrophilic channel of 1. Water escapes at 150 °C but is re-absorbed using water vapor. The reversible formation of this water cluster was supported from powder X-ray diffraction (PXRD), FTIR spectra and TGA/DTA. The reversible dehydration/rehydration behaviors enable analysis of the nature of the intermolecular interactions that occur between the planar hexameric water and the resulting host framework. When the auxiliary ligand bpe was removed from the synthetic conditions, another complex {[Cu(btec)0.5(H2O)3]⋅H2O}n (2) only encapsulating a water dimer was afforded, which suggests bpe plays an important role in the formation of the framework and water clusters.
Graphical abstractThe reversible formation of a centrosymmetric planar water hexamer is within the crystal host of {[Cu(bpe)(btec)0.5(H2O)2]⋅(H2O)4}n (1).Figure optionsDownload full-size imageDownload as PowerPoint slide