| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1402030 | Journal of Molecular Structure | 2015 | 13 Pages |
•CT complexes of two macrocyclic crown ethers were reported.•Bonding modes were ascertained from various spectral techniques.•Complexes were characterized in both solution and solid state.•Spectroscopic, thermal and kinetic properties were determined.•Microstructure properties of the reported complexes were observed.
The study of the complexing ability of macrocyclic compounds to organic and inorganic substances is of great interest. The aim of this work is to provide basic data that can be used to the assessment of macrocyclic crown ethers quantitatively based on charge-transfer (CT) complexation. This goal was achieved by preparing CT complexes of two interesting mixed nitrogen–oxygen crown ethers with acido acceptors (chloranilic and picric acid), which were fully structurally characterized. The crown ethers are 4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane (HDHC) and 1,4,10-trioxa-7,13-diaza-cyclopentadecane (TDPD). The obtained complexes were structurally characterized via elemental analysis, IR, Raman, 1H NMR, and UV–visible spectroscopy. Thermal properties of these complexes were also studied, and their kinetic thermodynamic parameters were calculated. Furthermore, the microstructure properties of these complexes have also been investigated using X-ray diffraction (XRD) and scanning electron microscope (SEM).
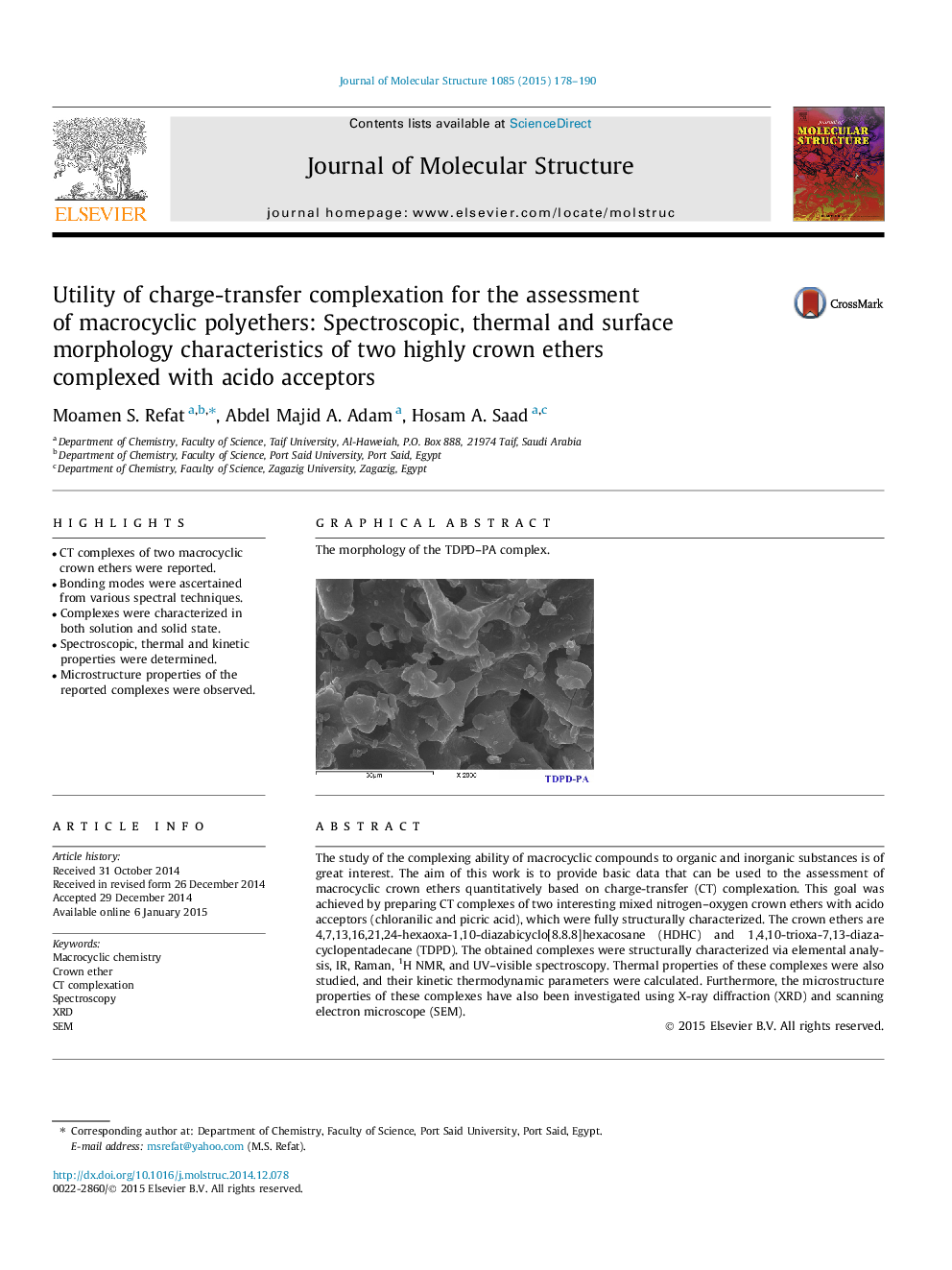
Graphical abstractThe morphology of the TDPD–PA complex.Figure optionsDownload full-size imageDownload as PowerPoint slide