| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1402046 | Journal of Molecular Structure | 2015 | 6 Pages |
•Three dimethoxy-substituted luminol derivatives are investigated.•Their structures, electron and spectrum properties are compared.•A derivative with high chemiluminescent efficiency is suggested.
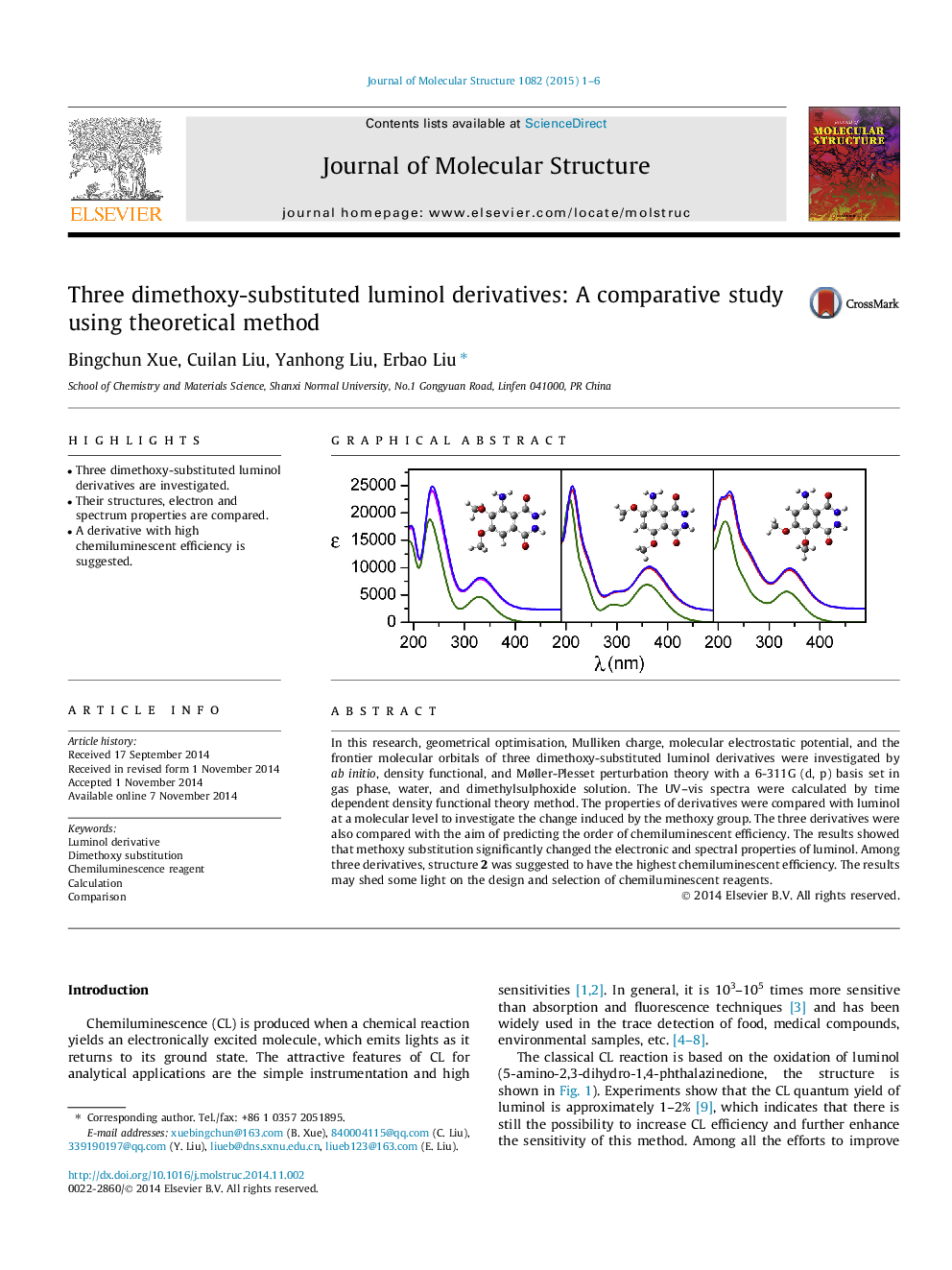
In this research, geometrical optimisation, Mulliken charge, molecular electrostatic potential, and the frontier molecular orbitals of three dimethoxy-substituted luminol derivatives were investigated by ab initio, density functional, and Møller-Plesset perturbation theory with a 6-311G (d, p) basis set in gas phase, water, and dimethylsulphoxide solution. The UV–vis spectra were calculated by time dependent density functional theory method. The properties of derivatives were compared with luminol at a molecular level to investigate the change induced by the methoxy group. The three derivatives were also compared with the aim of predicting the order of chemiluminescent efficiency. The results showed that methoxy substitution significantly changed the electronic and spectral properties of luminol. Among three derivatives, structure 2 was suggested to have the highest chemiluminescent efficiency. The results may shed some light on the design and selection of chemiluminescent reagents.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide