| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1402057 | Journal of Molecular Structure | 2015 | 6 Pages |
•New chiral and achiral ligands based on hexasubstituted benzene scaffold starting from phloroglucinol were prepared.•The chiral ligand formed stable complexes with amino acid derivatives.•The structures of the ligand and its complexes were confirmed both by experimental (NMR, IR) and computational studies.
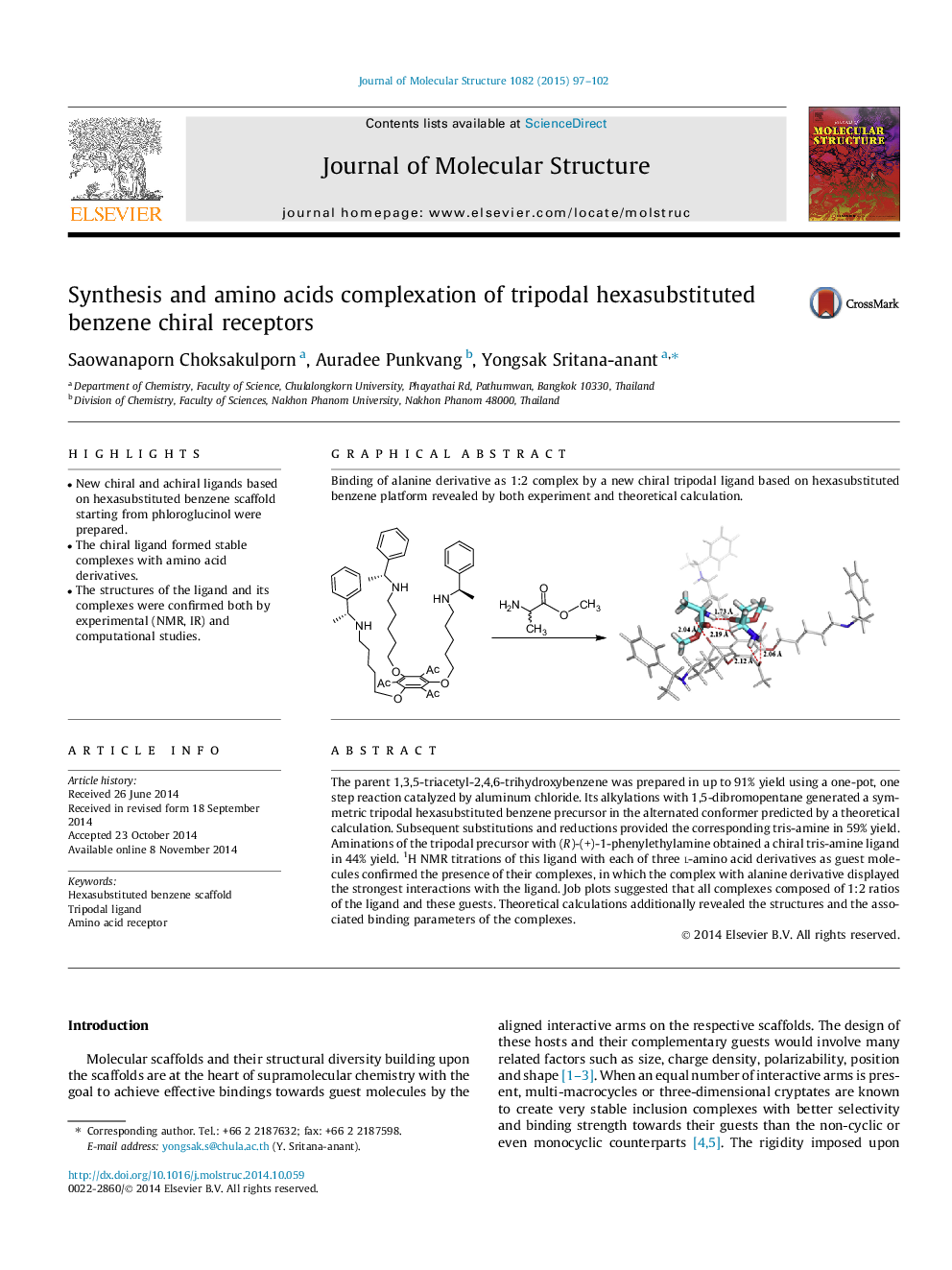
The parent 1,3,5-triacetyl-2,4,6-trihydroxybenzene was prepared in up to 91% yield using a one-pot, one step reaction catalyzed by aluminum chloride. Its alkylations with 1,5-dibromopentane generated a symmetric tripodal hexasubstituted benzene precursor in the alternated conformer predicted by a theoretical calculation. Subsequent substitutions and reductions provided the corresponding tris-amine in 59% yield. Aminations of the tripodal precursor with (R)-(+)-1-phenylethylamine obtained a chiral tris-amine ligand in 44% yield. 1H NMR titrations of this ligand with each of three l-amino acid derivatives as guest molecules confirmed the presence of their complexes, in which the complex with alanine derivative displayed the strongest interactions with the ligand. Job plots suggested that all complexes composed of 1:2 ratios of the ligand and these guests. Theoretical calculations additionally revealed the structures and the associated binding parameters of the complexes.
Graphical abstractBinding of alanine derivative as 1:2 complex by a new chiral tripodal ligand based on hexasubstituted benzene platform revealed by both experiment and theoretical calculation.Figure optionsDownload full-size imageDownload as PowerPoint slide