| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1402117 | Journal of Molecular Structure | 2015 | 7 Pages |
•One dimensional coordination polymer of Cu(II) with aminopyridine and chelidamic acid is X-ray characterized.•We report a density functional study of the energetic features of several noncovalent interactions.•We perform an “atoms-in-molecules” analysis to investigate the close intermolecular contacts.
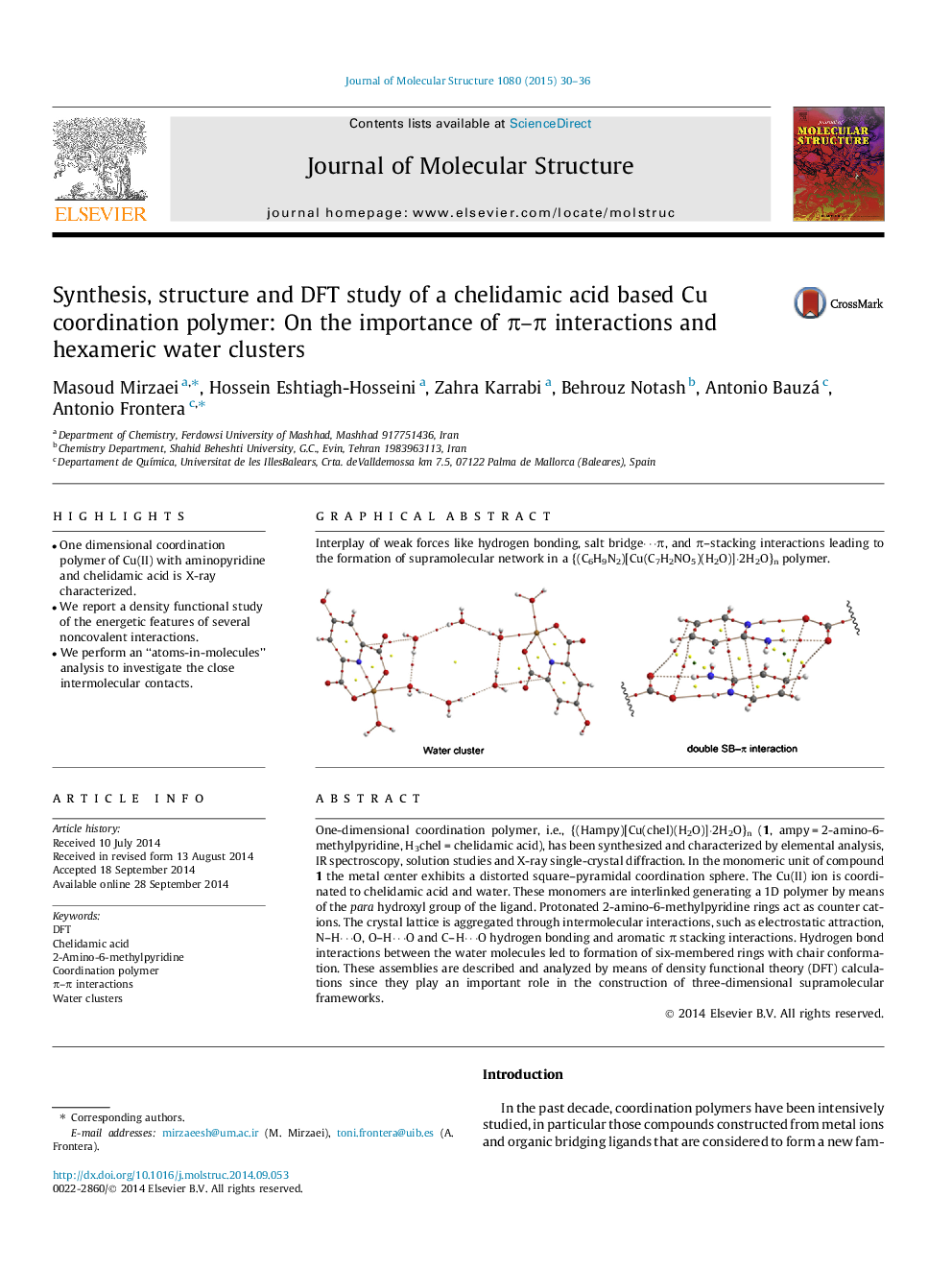
One-dimensional coordination polymer, i.e., {(Hampy)[Cu(chel)(H2O)]⋅2H2O}n (1, ampy = 2-amino-6-methylpyridine, H3chel = chelidamic acid), has been synthesized and characterized by elemental analysis, IR spectroscopy, solution studies and X-ray single-crystal diffraction. In the monomeric unit of compound 1 the metal center exhibits a distorted square–pyramidal coordination sphere. The Cu(II) ion is coordinated to chelidamic acid and water. These monomers are interlinked generating a 1D polymer by means of the para hydroxyl group of the ligand. Protonated 2-amino-6-methylpyridine rings act as counter cations. The crystal lattice is aggregated through intermolecular interactions, such as electrostatic attraction, N–H⋯O, O–H⋯O and C–H⋯O hydrogen bonding and aromatic π stacking interactions. Hydrogen bond interactions between the water molecules led to formation of six-membered rings with chair conformation. These assemblies are described and analyzed by means of density functional theory (DFT) calculations since they play an important role in the construction of three-dimensional supramolecular frameworks.
Graphical abstractInterplay of weak forces like hydrogen bonding, salt bridge⋯π, and π–stacking interactions leading to the formation of supramolecular network in a {(C6H9N2)[Cu(C7H2NO5)(H2O)]⋅2H2O}n polymer.Figure optionsDownload full-size imageDownload as PowerPoint slide