| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1402131 | Journal of Molecular Structure | 2015 | 8 Pages |
•New hydrazono-thiazolidin-4-ones with N-methyl carbazole pendant are synthesized.•X-ray diffraction of representative thizolidin-4-one has been reported.•The results of DFT studies on tautomers are correlated with experimental results.•Compounds have been characterized by analytical and spectral data.•Photophysical properties were studied by means of UV/vis and fluorescence.
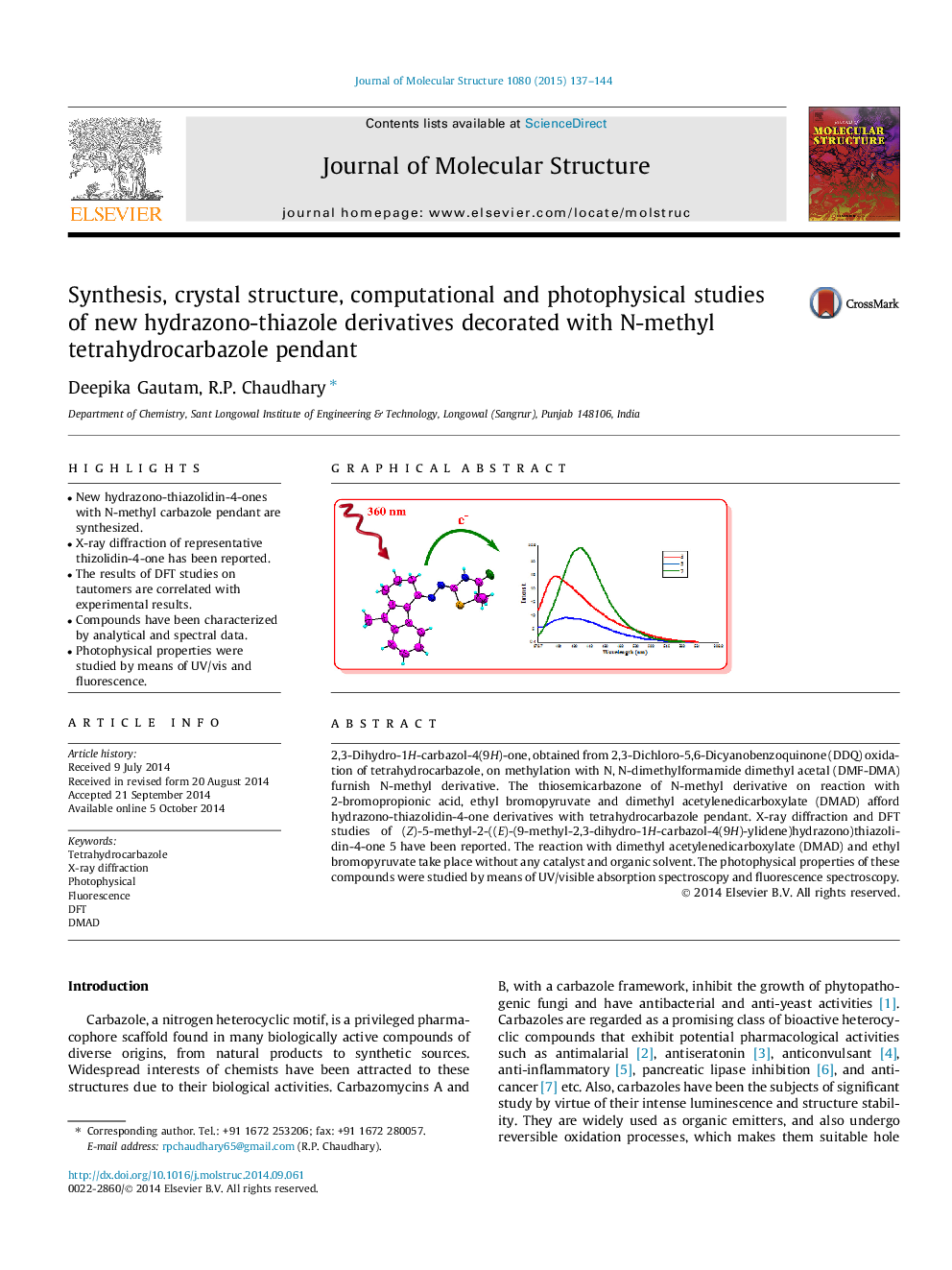
2,3-Dihydro-1H-carbazol-4(9H)-one, obtained from 2,3-Dichloro-5,6-Dicyanobenzoquinone (DDQ) oxidation of tetrahydrocarbazole, on methylation with N, N-dimethylformamide dimethyl acetal (DMF-DMA) furnish N-methyl derivative. The thiosemicarbazone of N-methyl derivative on reaction with 2-bromopropionic acid, ethyl bromopyruvate and dimethyl acetylenedicarboxylate (DMAD) afford hydrazono-thiazolidin-4-one derivatives with tetrahydrocarbazole pendant. X-ray diffraction and DFT studies of (Z)-5-methyl-2-((E)-(9-methyl-2,3-dihydro-1H-carbazol-4(9H)-ylidene)hydrazono)thiazolidin-4-one 5 have been reported. The reaction with dimethyl acetylenedicarboxylate (DMAD) and ethyl bromopyruvate take place without any catalyst and organic solvent. The photophysical properties of these compounds were studied by means of UV/visible absorption spectroscopy and fluorescence spectroscopy.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide