| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1402147 | Journal of Molecular Structure | 2015 | 7 Pages |
•Two new complexes were hydrolytically synthesized in aqueous solutions at basic pH.•Crystal structures are identified as Na3[Ln(Pydc)3]·14H2O.•Coordination polyhedron is a tricapped trigonal prism with O atoms in the corners.•Mononuclear rare-earth ions display paramagnetic behavior.
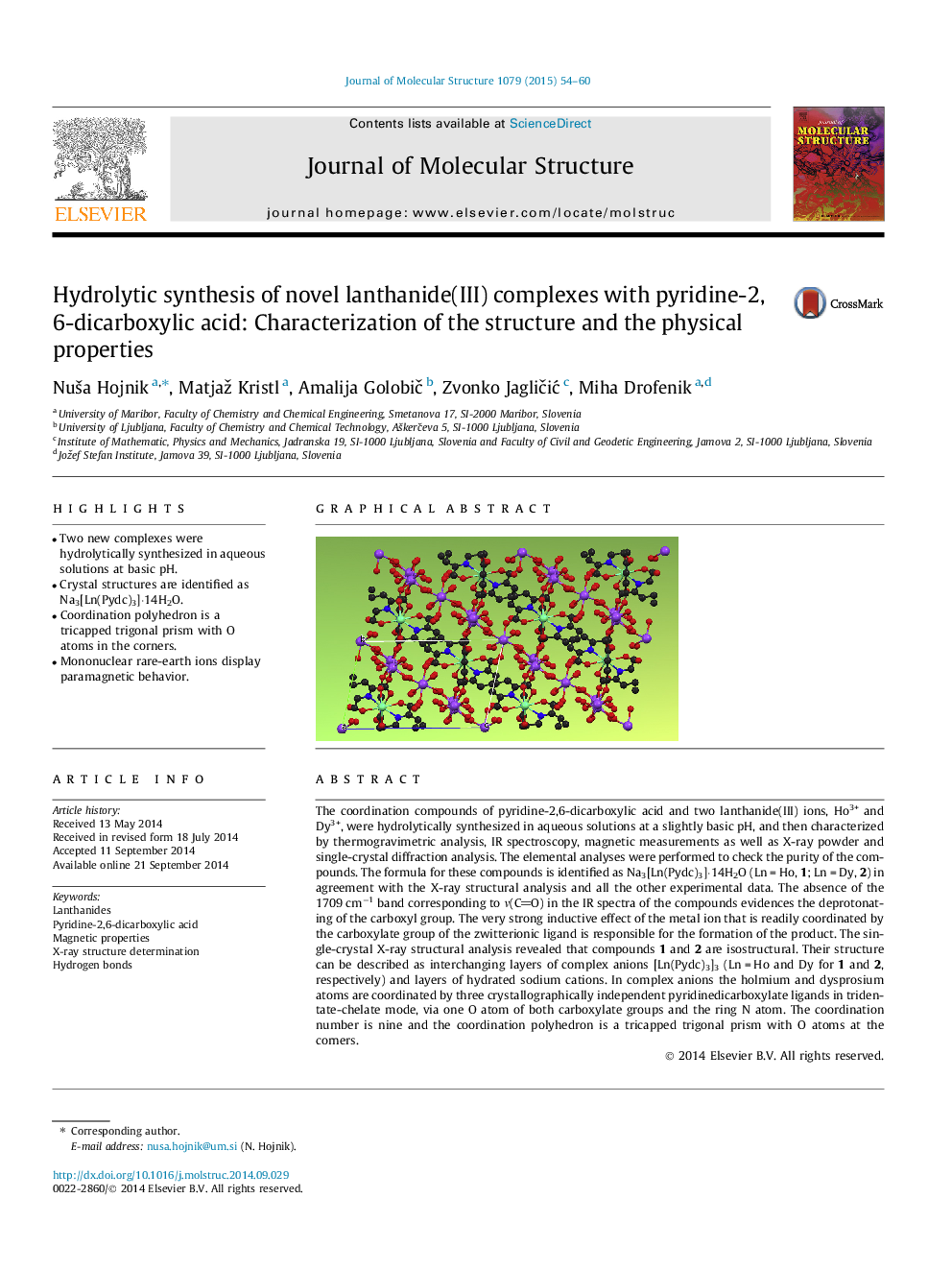
The coordination compounds of pyridine-2,6-dicarboxylic acid and two lanthanide(III) ions, Ho3+ and Dy3+, were hydrolytically synthesized in aqueous solutions at a slightly basic pH, and then characterized by thermogravimetric analysis, IR spectroscopy, magnetic measurements as well as X-ray powder and single-crystal diffraction analysis. The elemental analyses were performed to check the purity of the compounds. The formula for these compounds is identified as Na3[Ln(Pydc)3]⋅14H2O (Ln = Ho, 1; Ln = Dy, 2) in agreement with the X-ray structural analysis and all the other experimental data. The absence of the 1709 cm−1 band corresponding to ν(СO) in the IR spectra of the compounds evidences the deprotonating of the carboxyl group. The very strong inductive effect of the metal ion that is readily coordinated by the carboxylate group of the zwitterionic ligand is responsible for the formation of the product. The single-crystal X-ray structural analysis revealed that compounds 1 and 2 are isostructural. Their structure can be described as interchanging layers of complex anions [Ln(Pydc)3]3 (Ln = Ho and Dy for 1 and 2, respectively) and layers of hydrated sodium cations. In complex anions the holmium and dysprosium atoms are coordinated by three crystallographically independent pyridinedicarboxylate ligands in tridentate-chelate mode, via one O atom of both carboxylate groups and the ring N atom. The coordination number is nine and the coordination polyhedron is a tricapped trigonal prism with O atoms at the corners.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide