| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1402151 | Journal of Molecular Structure | 2015 | 4 Pages |
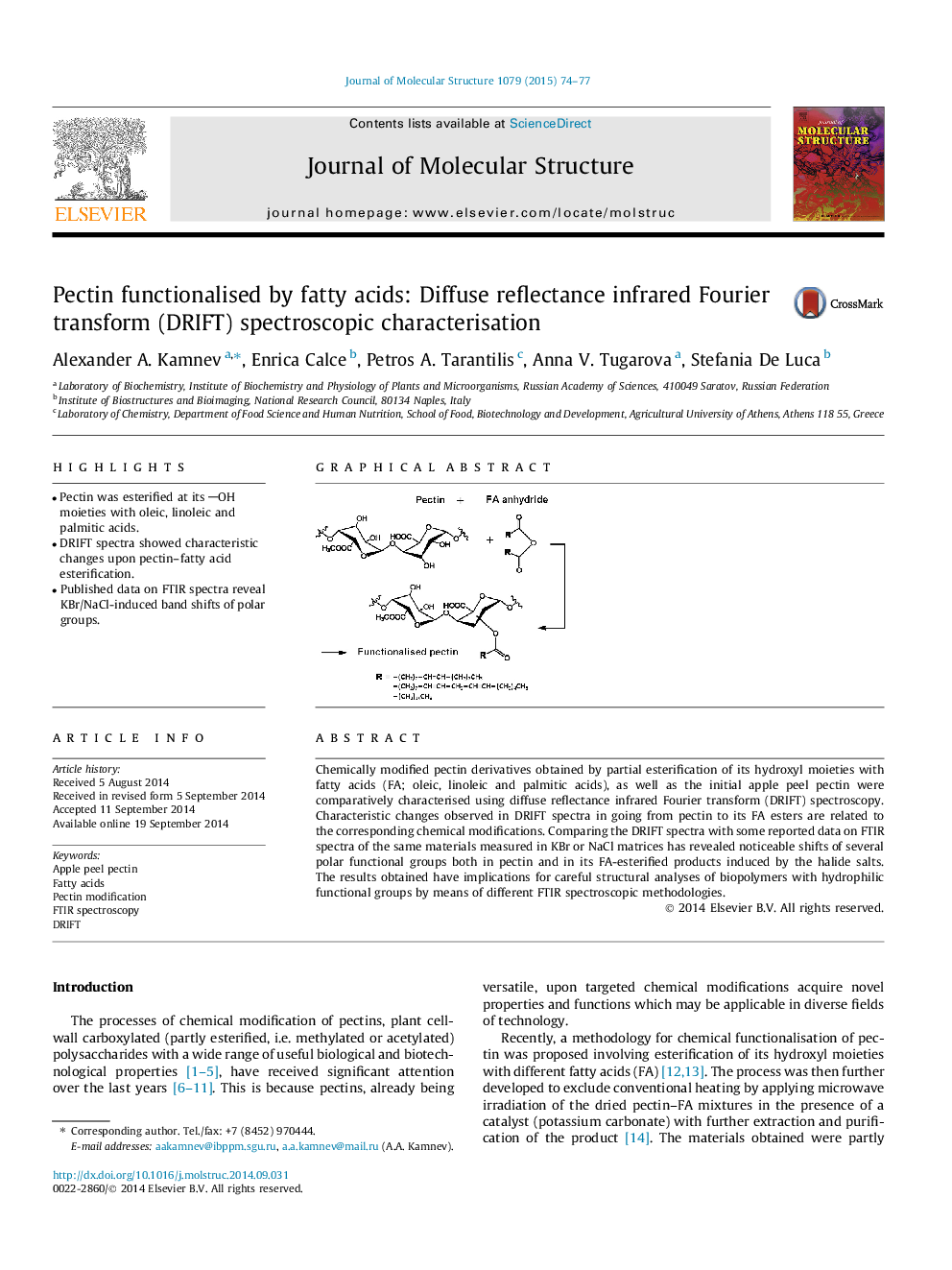
•Pectin was esterified at its OH moieties with oleic, linoleic and palmitic acids.•DRIFT spectra showed characteristic changes upon pectin–fatty acid esterification.•Published data on FTIR spectra reveal KBr/NaCl-induced band shifts of polar groups.
Chemically modified pectin derivatives obtained by partial esterification of its hydroxyl moieties with fatty acids (FA; oleic, linoleic and palmitic acids), as well as the initial apple peel pectin were comparatively characterised using diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy. Characteristic changes observed in DRIFT spectra in going from pectin to its FA esters are related to the corresponding chemical modifications. Comparing the DRIFT spectra with some reported data on FTIR spectra of the same materials measured in KBr or NaCl matrices has revealed noticeable shifts of several polar functional groups both in pectin and in its FA-esterified products induced by the halide salts. The results obtained have implications for careful structural analyses of biopolymers with hydrophilic functional groups by means of different FTIR spectroscopic methodologies.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide