| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1402204 | Journal of Molecular Structure | 2015 | 8 Pages |
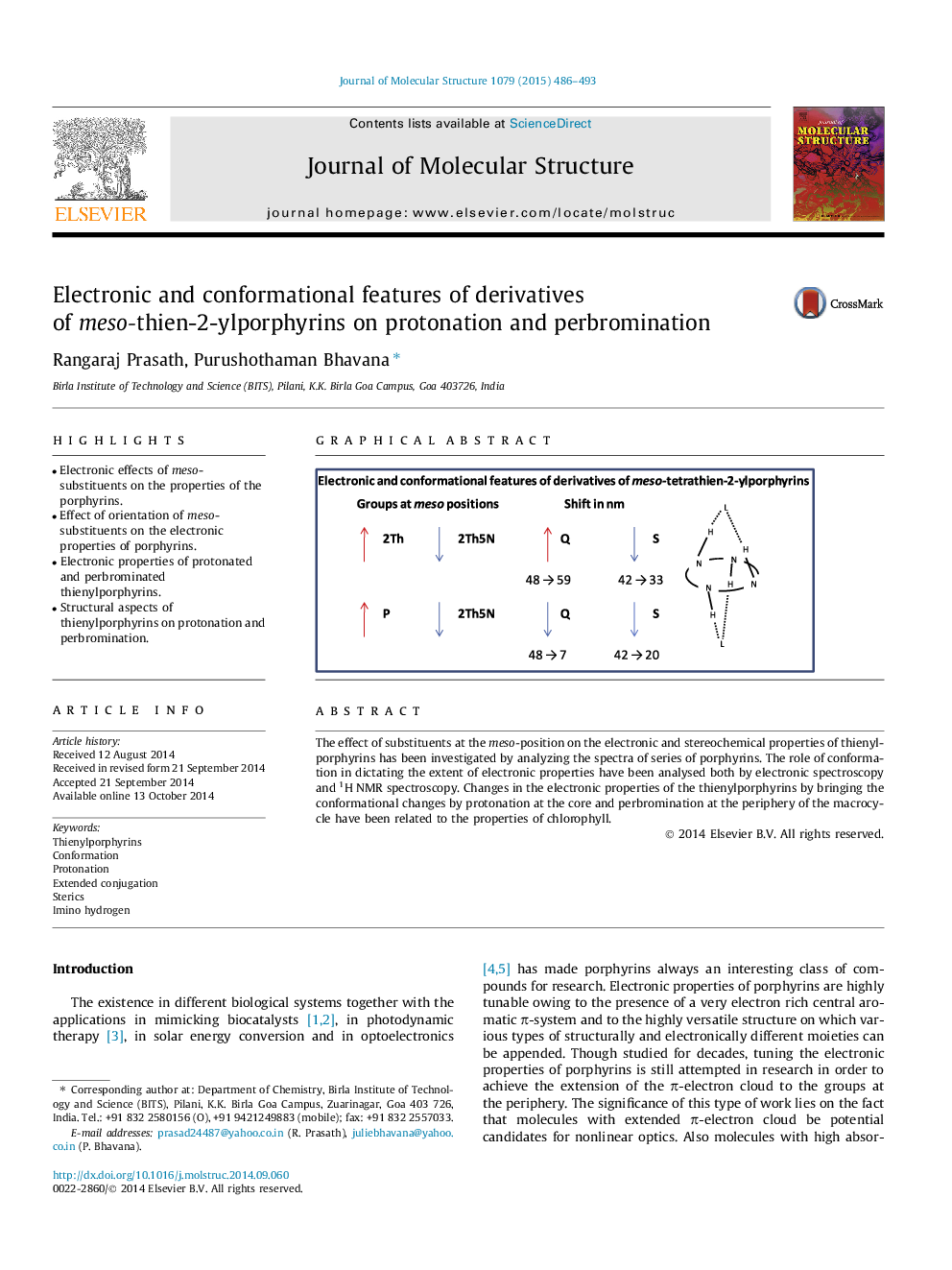
•Electronic effects of meso-substituents on the properties of the porphyrins.•Effect of orientation of meso-substituents on the electronic properties of porphyrins.•Electronic properties of protonated and perbrominated thienylporphyrins.•Structural aspects of thienylporphyrins on protonation and perbromination.
The effect of substituents at the meso-position on the electronic and stereochemical properties of thienylporphyrins has been investigated by analyzing the spectra of series of porphyrins. The role of conformation in dictating the extent of electronic properties have been analysed both by electronic spectroscopy and 1H NMR spectroscopy. Changes in the electronic properties of the thienylporphyrins by bringing the conformational changes by protonation at the core and perbromination at the periphery of the macrocycle have been related to the properties of chlorophyll.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide