| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1402269 | Journal of Molecular Structure | 2014 | 14 Pages |
•Hydrazone complexes.•The DFT and chemical reactivity.•Spectroscopic characterization.•Studying the antimicrobial, cytotoxicity and antioxidant activities.
Hydrazone complexes of Co(II), Ni(II), Cu(II), Pd(II), Zn(II) and U(VI)O2 with (E)-3-(2-(2-hydroxybenzylidene)hydrazinyl)-3-oxo-N-(p-tolyl)propanamide (H2SHAH) were synthesized. The complex structure was elucidated by analysis (elemental and thermal), spectroscopy (1H NMR, IR, UV–visible, MS) and physical measurements (magnetic susceptibility and molar conductance). The kinetic and thermodynamic parameters for the different decomposition steps of some complexes were calculated using the Coats–Redfern and Horowitz–Metzger methods. Also, the bond length, bond angle, HOMO, LUMO, dipole moment and charges on the atoms were estimated to confirm the geometry of the ligand and the investigated complexes. Moreover, antimicrobial activity of ligand and its complexes was tested against gram positive bacteria Staphylococcus aureus, gram-negative bacteria; Escherichia coli and pathogenic fungi; Candida albicans by using MICs method. Moreover, the antioxidant of the isolated compounds was studied by using the ABTS method. Finally, the compounds were also screened for antitumor study against HePG2 liver cells.
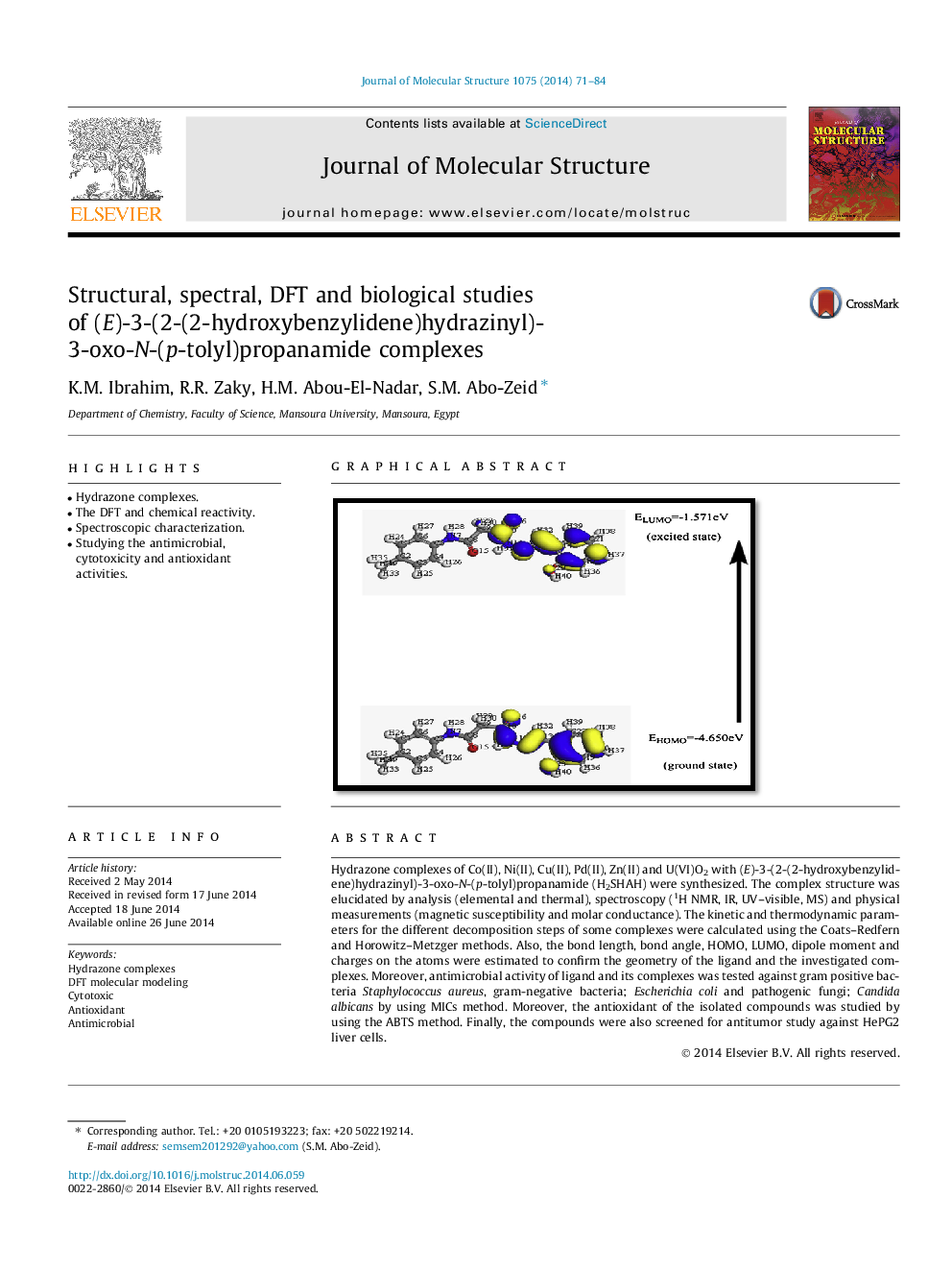
Graphical abstract3D plots Frontier orbital energies using DFT method for H2SHAH.Figure optionsDownload full-size imageDownload as PowerPoint slide