| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1402635 | Journal of Molecular Structure | 2014 | 8 Pages |
•Five Schiff bases were examined using NMR in solution, two of them were studied by CPMAS NMR.•Two Schiff base complexes were studied in solution and solid state by NMR.•The structures of two Schiff base ligands were defined using X-ray method.•The positions of tautomeric equilibrium were defined for all ligands.•The comparison of X-ray and NMR data has been done.
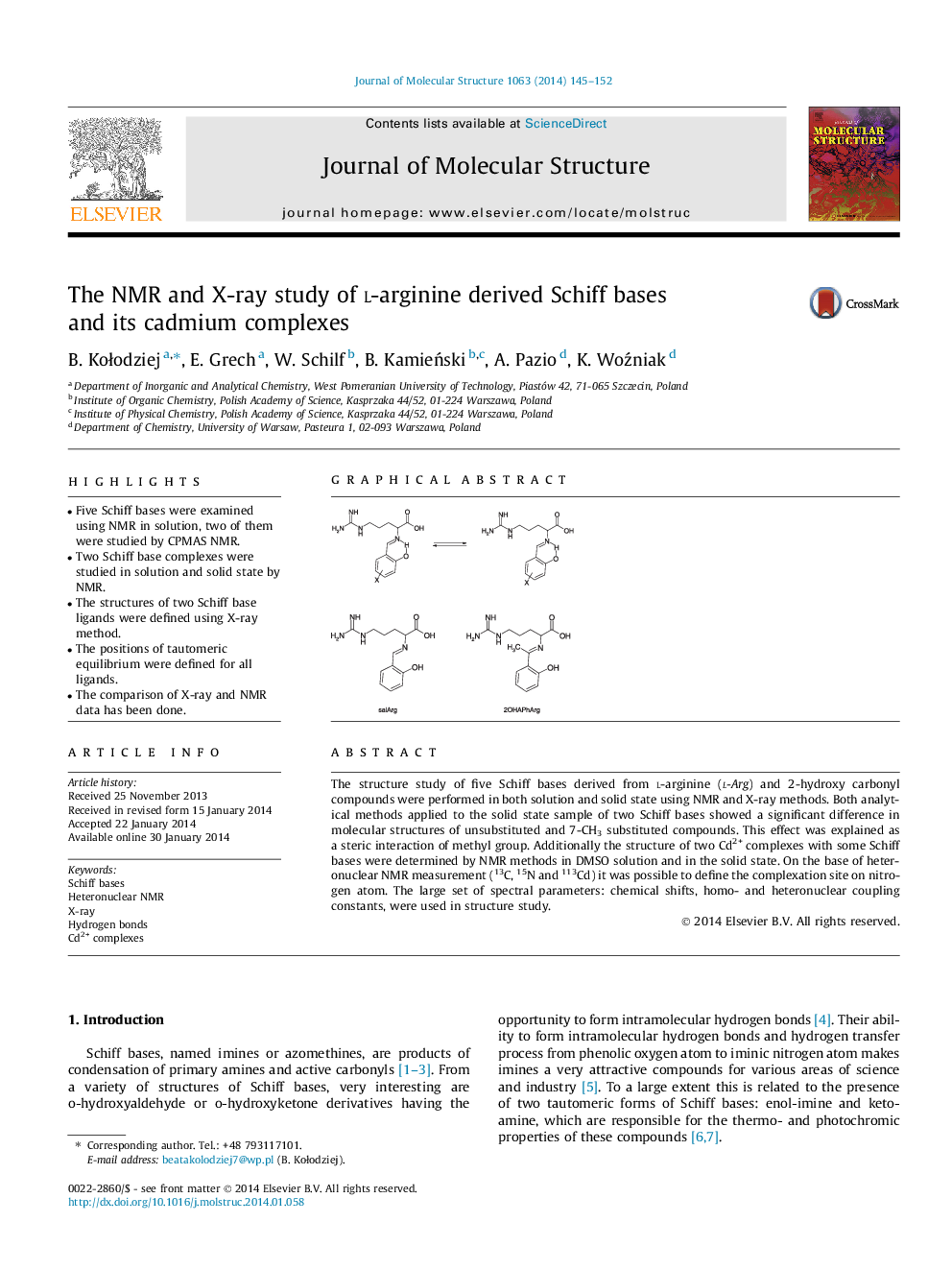
The structure study of five Schiff bases derived from l-arginine (l-Arg) and 2-hydroxy carbonyl compounds were performed in both solution and solid state using NMR and X-ray methods. Both analytical methods applied to the solid state sample of two Schiff bases showed a significant difference in molecular structures of unsubstituted and 7-CH3 substituted compounds. This effect was explained as a steric interaction of methyl group. Additionally the structure of two Cd2+ complexes with some Schiff bases were determined by NMR methods in DMSO solution and in the solid state. On the base of heteronuclear NMR measurement (13C, 15N and 113Cd) it was possible to define the complexation site on nitrogen atom. The large set of spectral parameters: chemical shifts, homo- and heteronuclear coupling constants, were used in structure study.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide