| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1402773 | Journal of Molecular Structure | 2014 | 9 Pages |
•Syntheses of disubstituted isophthalamide and pyridine-2,6dicarboxamide derivatives.•Structures of these derivatives and complexes with anions were obtained.•Associations of these derivatives with anions were studied.•Anions, F−, Cl−, Br−, H2PO4–, HSO4– and NO3– were H2PO4-, HSO4- and NO3- were selected.•Association constants and association energies of these complexes were obtained.
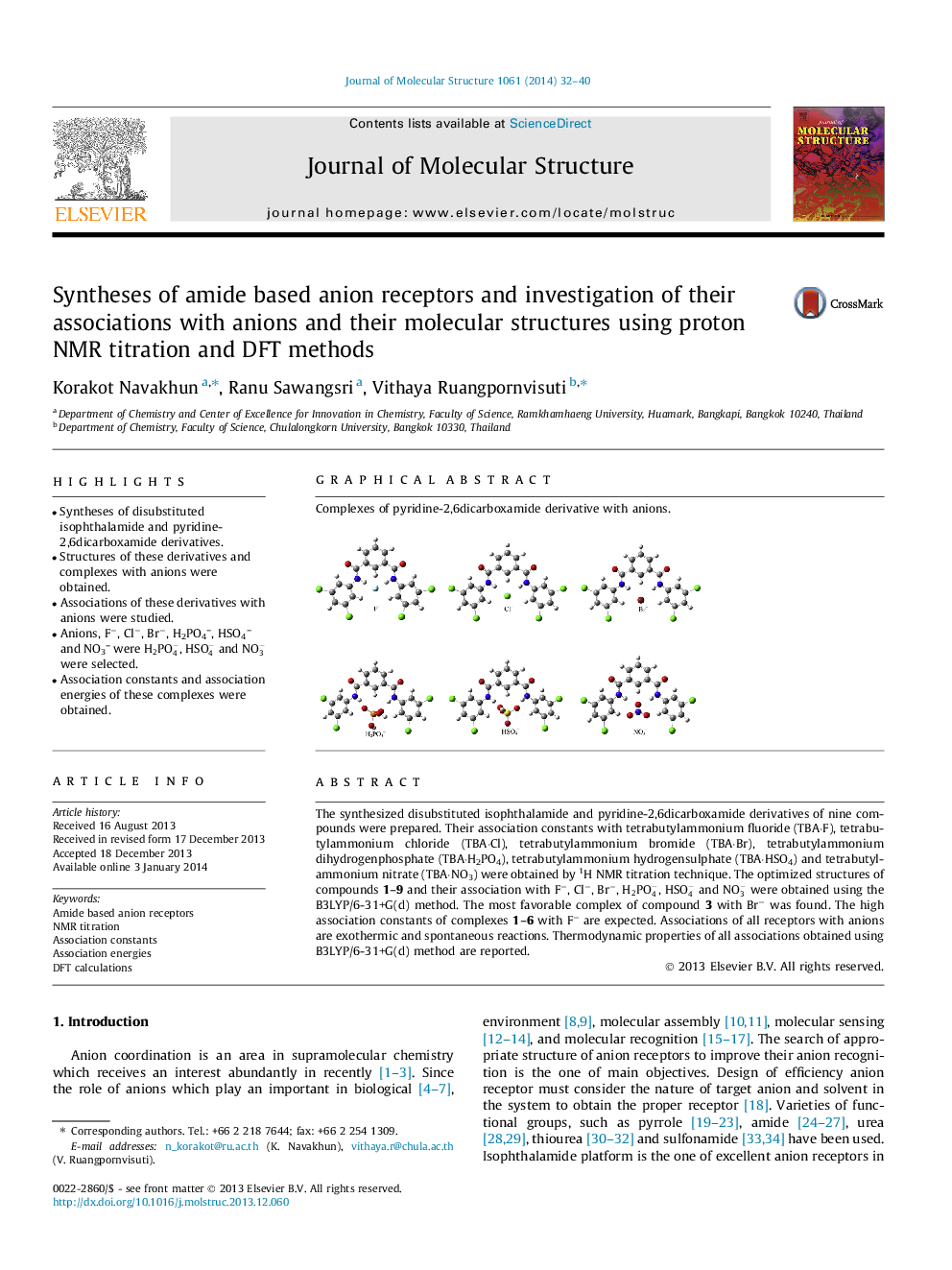
The synthesized disubstituted isophthalamide and pyridine-2,6dicarboxamide derivatives of nine compounds were prepared. Their association constants with tetrabutylammonium fluoride (TBA·F), tetrabutylammonium chloride (TBA·Cl), tetrabutylammonium bromide (TBA·Br), tetrabutylammonium dihydrogenphosphate (TBA·H2PO4), tetrabutylammonium hydrogensulphate (TBA·HSO4) and tetrabutylammonium nitrate (TBA·NO3) were obtained by 1H NMR titration technique. The optimized structures of compounds 1–9 and their association with F−, Cl−, Br−, H2PO4-, HSO4- and NO3- were obtained using the B3LYP/6-31+G(d) method. The most favorable complex of compound 3 with Br− was found. The high association constants of complexes 1–6 with F− are expected. Associations of all receptors with anions are exothermic and spontaneous reactions. Thermodynamic properties of all associations obtained using B3LYP/6-31+G(d) method are reported.
Graphical abstractComplexes of pyridine-2,6dicarboxamide derivative with anions.Figure optionsDownload full-size imageDownload as PowerPoint slide