| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1402917 | Journal of Molecular Structure | 2013 | 5 Pages |
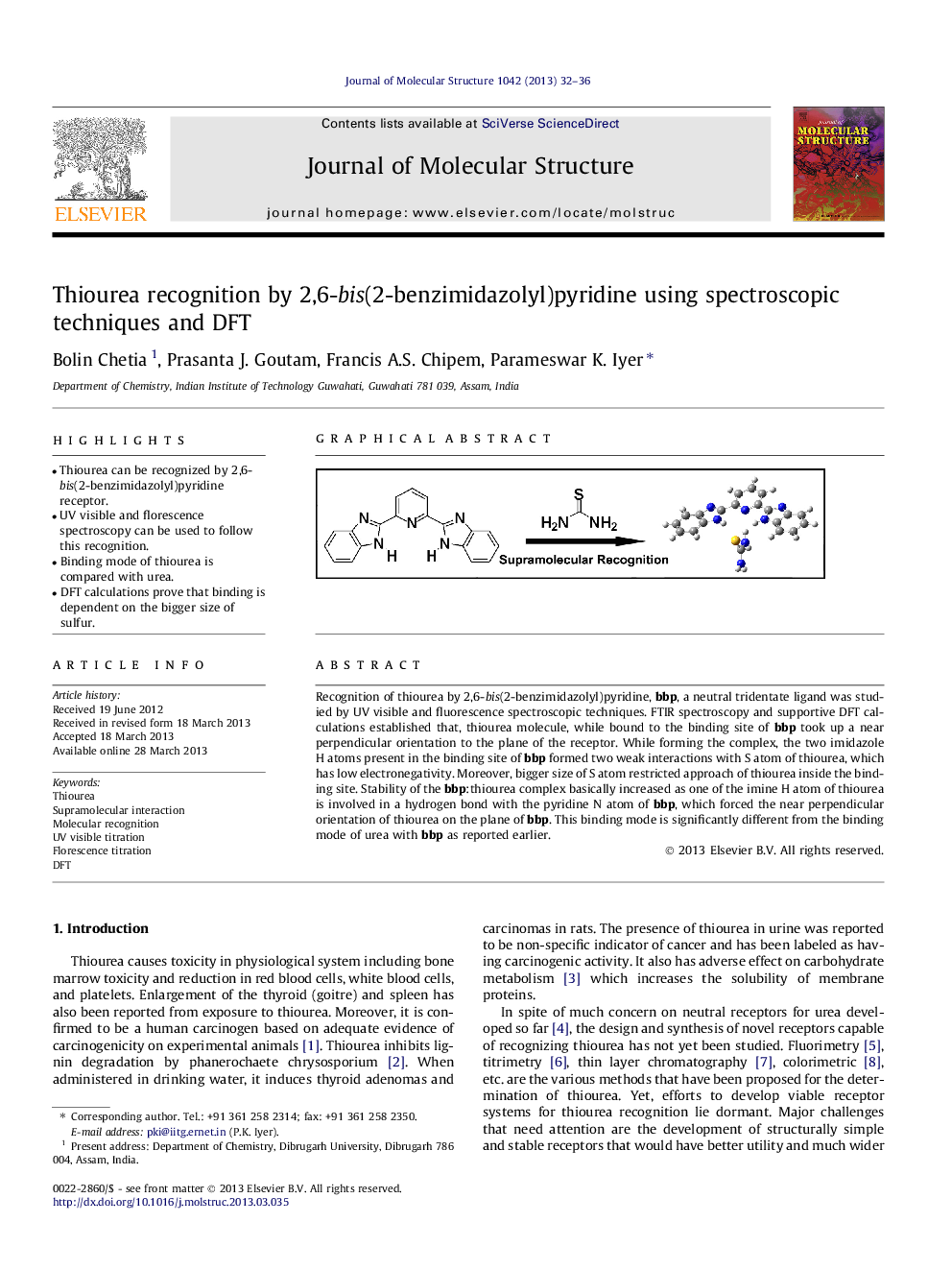
•Thiourea can be recognized by 2,6-bis(2-benzimidazolyl)pyridine receptor.•UV visible and florescence spectroscopy can be used to follow this recognition.•Binding mode of thiourea is compared with urea.•DFT calculations prove that binding is dependent on the bigger size of sulfur.
Recognition of thiourea by 2,6-bis(2-benzimidazolyl)pyridine, bbp, a neutral tridentate ligand was studied by UV visible and fluorescence spectroscopic techniques. FTIR spectroscopy and supportive DFT calculations established that, thiourea molecule, while bound to the binding site of bbp took up a near perpendicular orientation to the plane of the receptor. While forming the complex, the two imidazole H atoms present in the binding site of bbp formed two weak interactions with S atom of thiourea, which has low electronegativity. Moreover, bigger size of S atom restricted approach of thiourea inside the binding site. Stability of the bbp:thiourea complex basically increased as one of the imine H atom of thiourea is involved in a hydrogen bond with the pyridine N atom of bbp, which forced the near perpendicular orientation of thiourea on the plane of bbp. This binding mode is significantly different from the binding mode of urea with bbp as reported earlier.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide