| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1403064 | Journal of Molecular Structure | 2013 | 6 Pages |
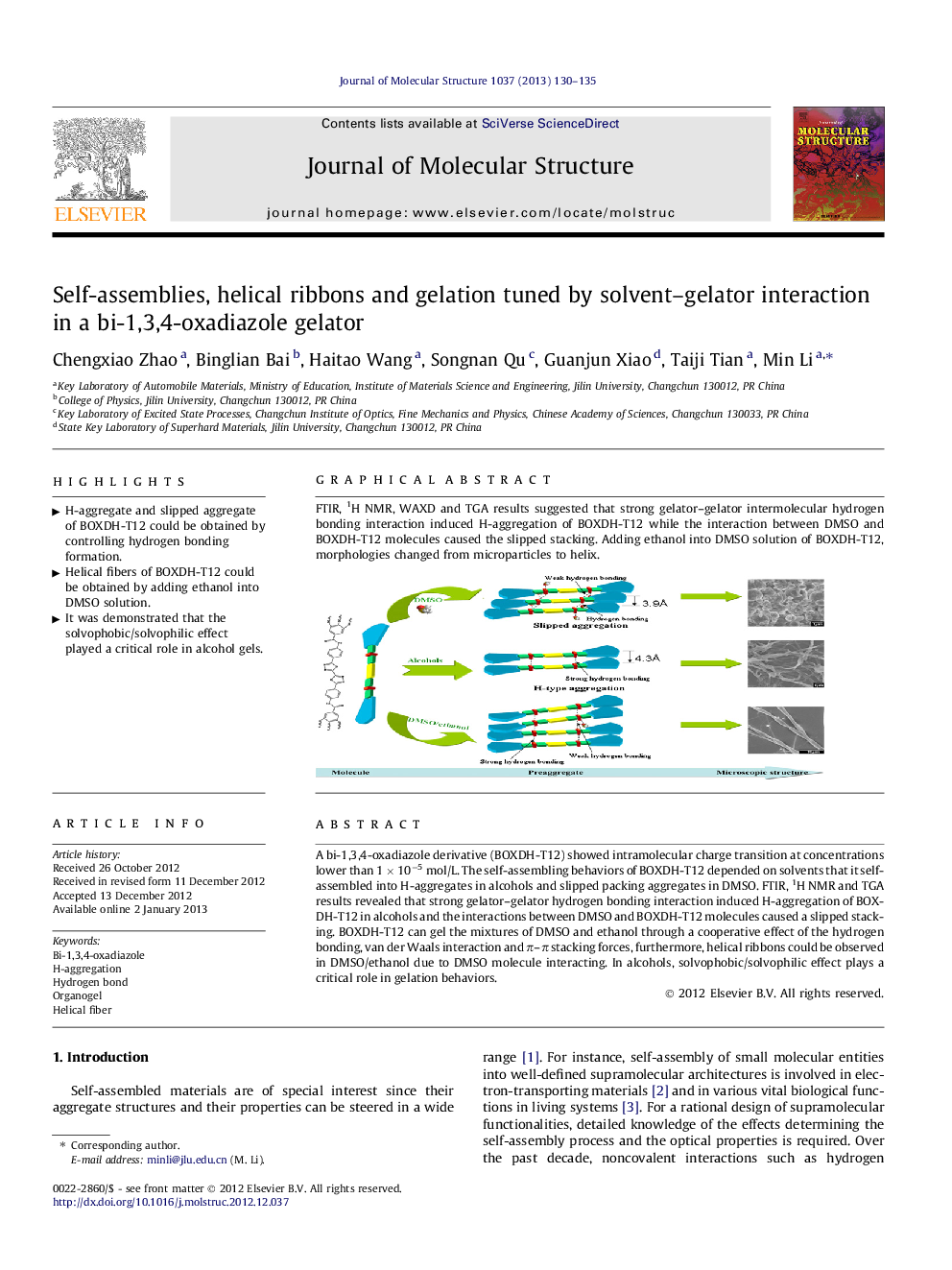
A bi-1,3,4-oxadiazole derivative (BOXDH-T12) showed intramolecular charge transition at concentrations lower than 1 × 10−5 mol/L. The self-assembling behaviors of BOXDH-T12 depended on solvents that it self-assembled into H-aggregates in alcohols and slipped packing aggregates in DMSO. FTIR, 1H NMR and TGA results revealed that strong gelator–gelator hydrogen bonding interaction induced H-aggregation of BOXDH-T12 in alcohols and the interactions between DMSO and BOXDH-T12 molecules caused a slipped stacking. BOXDH-T12 can gel the mixtures of DMSO and ethanol through a cooperative effect of the hydrogen bonding, van der Waals interaction and π–π stacking forces, furthermore, helical ribbons could be observed in DMSO/ethanol due to DMSO molecule interacting. In alcohols, solvophobic/solvophilic effect plays a critical role in gelation behaviors.
Graphical abstractFTIR, 1H NMR, WAXD and TGA results suggested that strong gelator–gelator intermolecular hydrogen bonding interaction induced H-aggregation of BOXDH-T12 while the interaction between DMSO and BOXDH-T12 molecules caused the slipped stacking. Adding ethanol into DMSO solution of BOXDH-T12, morphologies changed from microparticles to helix.Figure optionsDownload full-size imageDownload as PowerPoint slideHighlights► H-aggregate and slipped aggregate of BOXDH-T12 could be obtained by controlling hydrogen bonding formation. ► Helical fibers of BOXDH-T12 could be obtained by adding ethanol into DMSO solution. ► It was demonstrated that the solvophobic/solvophilic effect played a critical role in alcohol gels.