| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1404836 | Journal of Molecular Structure | 2015 | 12 Pages |
•The molecular structures of zwitterionic and neutral forms of quinolinic acid molecule are investigated.•Spectroscopic features of the compound are examined by UV, FT-IR, FT-Raman, 1H and 13C NMR techniques and DFT method.•Thermodynamic features, nonlinear optical properties and frontier molecular orbitals are calculated.
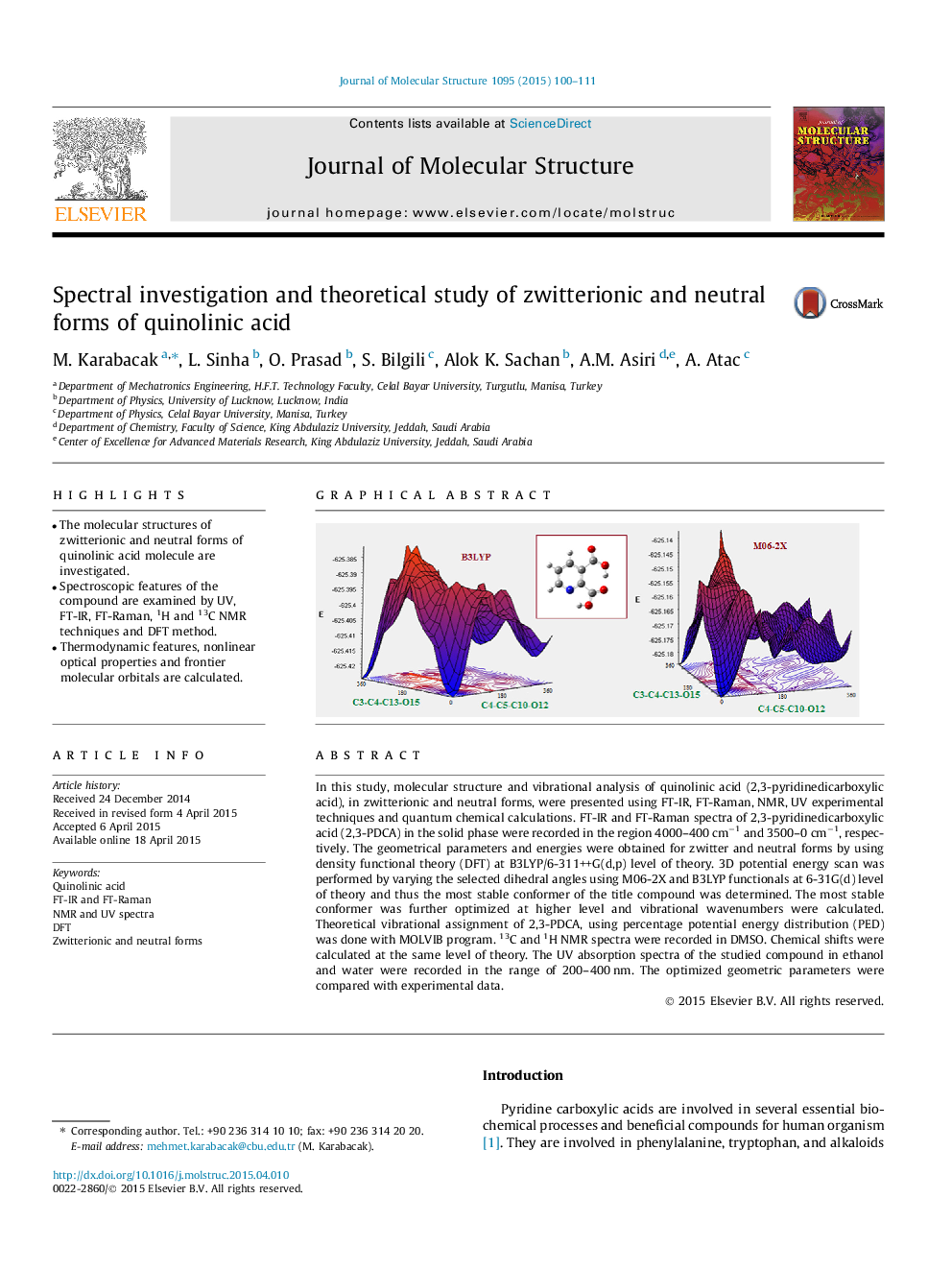
In this study, molecular structure and vibrational analysis of quinolinic acid (2,3-pyridinedicarboxylic acid), in zwitterionic and neutral forms, were presented using FT-IR, FT-Raman, NMR, UV experimental techniques and quantum chemical calculations. FT-IR and FT-Raman spectra of 2,3-pyridinedicarboxylic acid (2,3-PDCA) in the solid phase were recorded in the region 4000–400 cm−1 and 3500–0 cm−1, respectively. The geometrical parameters and energies were obtained for zwitter and neutral forms by using density functional theory (DFT) at B3LYP/6-311++G(d,p) level of theory. 3D potential energy scan was performed by varying the selected dihedral angles using M06-2X and B3LYP functionals at 6-31G(d) level of theory and thus the most stable conformer of the title compound was determined. The most stable conformer was further optimized at higher level and vibrational wavenumbers were calculated. Theoretical vibrational assignment of 2,3-PDCA, using percentage potential energy distribution (PED) was done with MOLVIB program. 13C and 1H NMR spectra were recorded in DMSO. Chemical shifts were calculated at the same level of theory. The UV absorption spectra of the studied compound in ethanol and water were recorded in the range of 200–400 nm. The optimized geometric parameters were compared with experimental data.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide