| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1404850 | Journal of Molecular Structure | 2015 | 6 Pages |
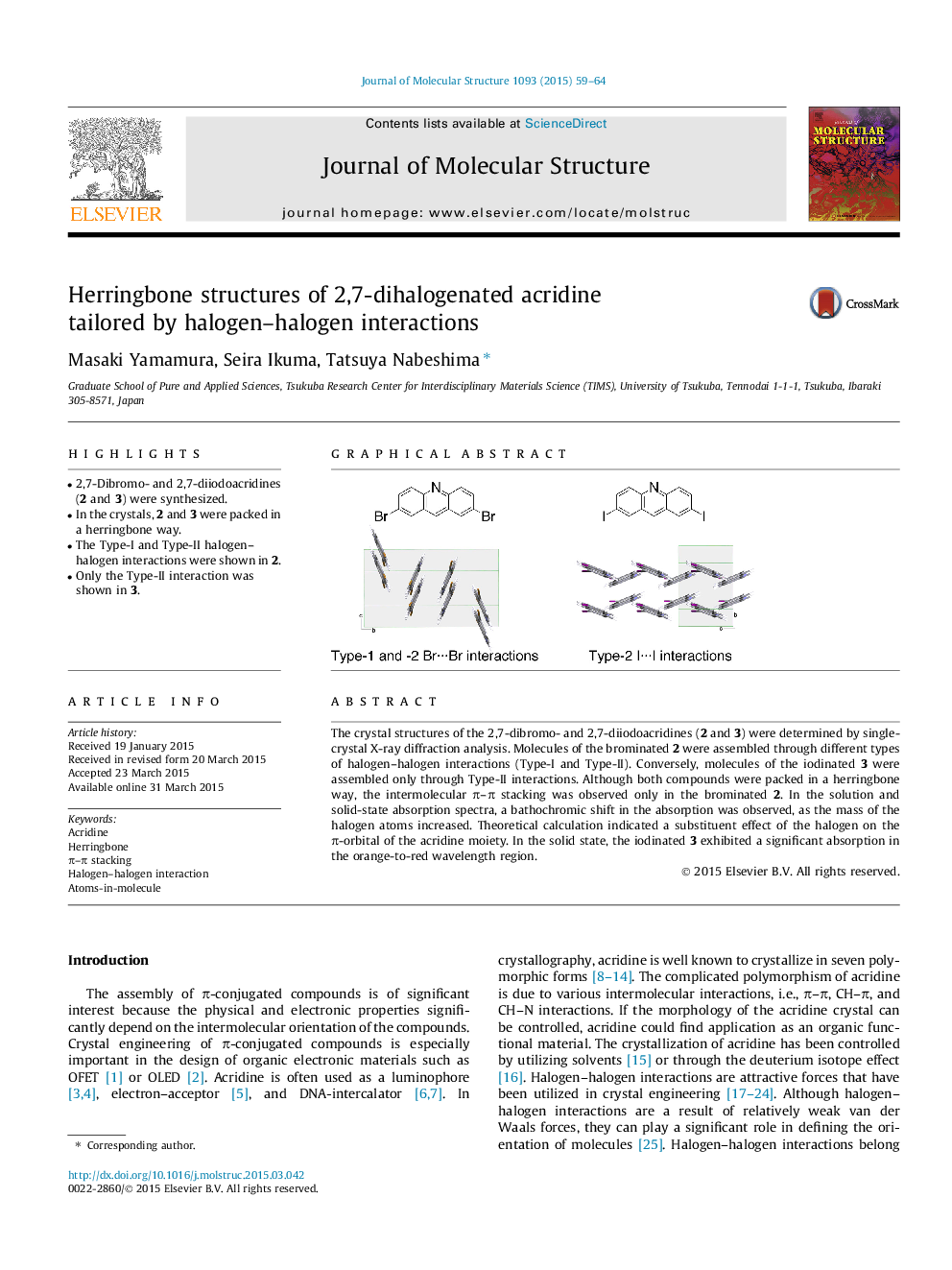
•2,7-Dibromo- and 2,7-diiodoacridines (2 and 3) were synthesized.•In the crystals, 2 and 3 were packed in a herringbone way.•The Type-I and Type-II halogen–halogen interactions were shown in 2.•Only the Type-II interaction was shown in 3.
The crystal structures of the 2,7-dibromo- and 2,7-diiodoacridines (2 and 3) were determined by single-crystal X-ray diffraction analysis. Molecules of the brominated 2 were assembled through different types of halogen–halogen interactions (Type-I and Type-II). Conversely, molecules of the iodinated 3 were assembled only through Type-II interactions. Although both compounds were packed in a herringbone way, the intermolecular π–π stacking was observed only in the brominated 2. In the solution and solid-state absorption spectra, a bathochromic shift in the absorption was observed, as the mass of the halogen atoms increased. Theoretical calculation indicated a substituent effect of the halogen on the π-orbital of the acridine moiety. In the solid state, the iodinated 3 exhibited a significant absorption in the orange-to-red wavelength region.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide