| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1404941 | Journal of Molecular Structure | 2015 | 7 Pages |
•Conformations of 2-pyridinecarboxylic acid are investigated by IR spectroscopy.•Stabilization due to intramolecular hydrogen bonding is examined by DFT method.•Conformational changes in argon matrices occur upon UV irradiation.•Photoreaction pathways are derived from kinetic analysis of IR absorbance changes.
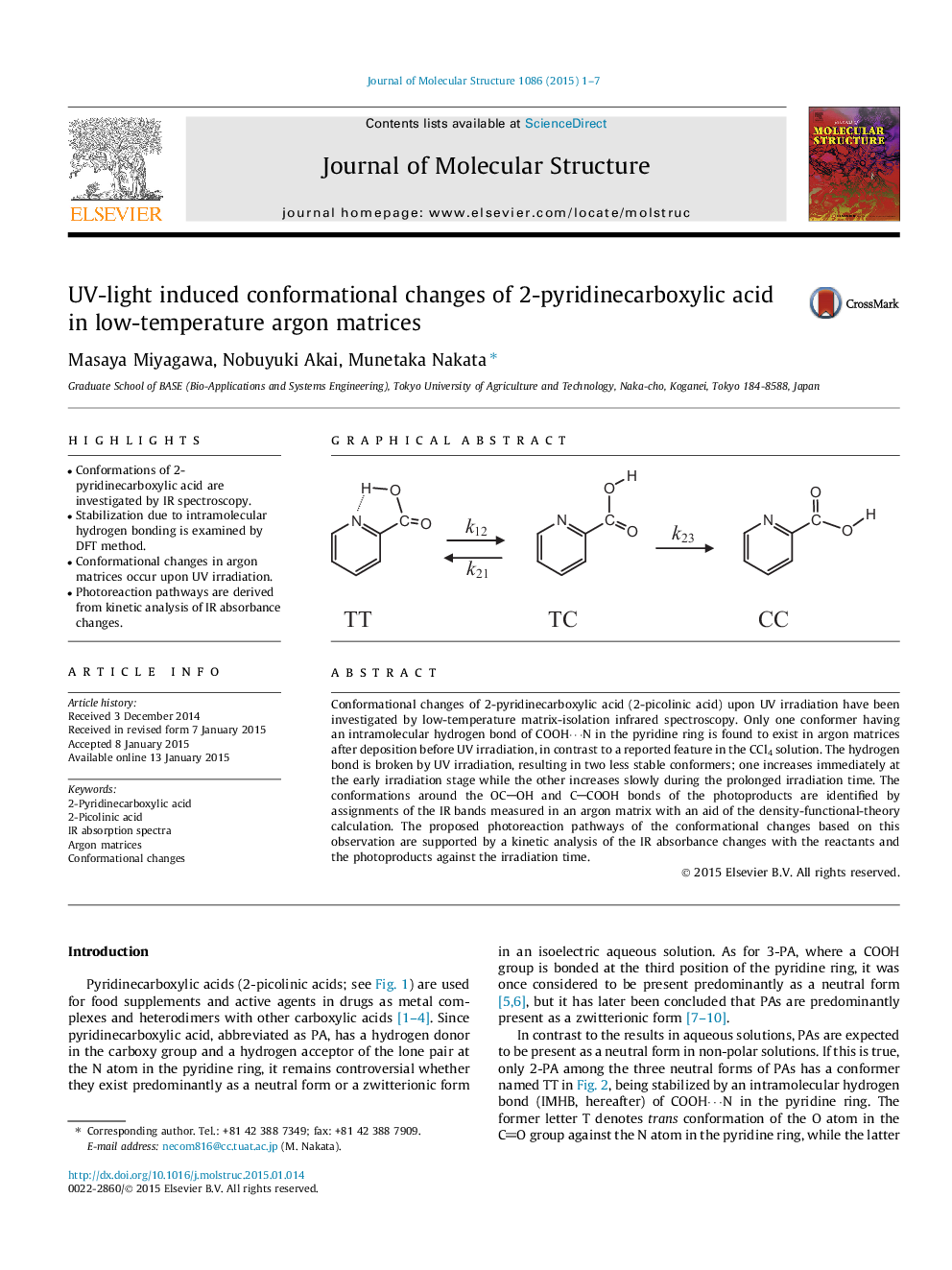
Conformational changes of 2-pyridinecarboxylic acid (2-picolinic acid) upon UV irradiation have been investigated by low-temperature matrix-isolation infrared spectroscopy. Only one conformer having an intramolecular hydrogen bond of COOH⋯N in the pyridine ring is found to exist in argon matrices after deposition before UV irradiation, in contrast to a reported feature in the CCl4 solution. The hydrogen bond is broken by UV irradiation, resulting in two less stable conformers; one increases immediately at the early irradiation stage while the other increases slowly during the prolonged irradiation time. The conformations around the OCOH and CCOOH bonds of the photoproducts are identified by assignments of the IR bands measured in an argon matrix with an aid of the density-functional-theory calculation. The proposed photoreaction pathways of the conformational changes based on this observation are supported by a kinetic analysis of the IR absorbance changes with the reactants and the photoproducts against the irradiation time.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide