| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1404950 | Journal of Molecular Structure | 2015 | 13 Pages |
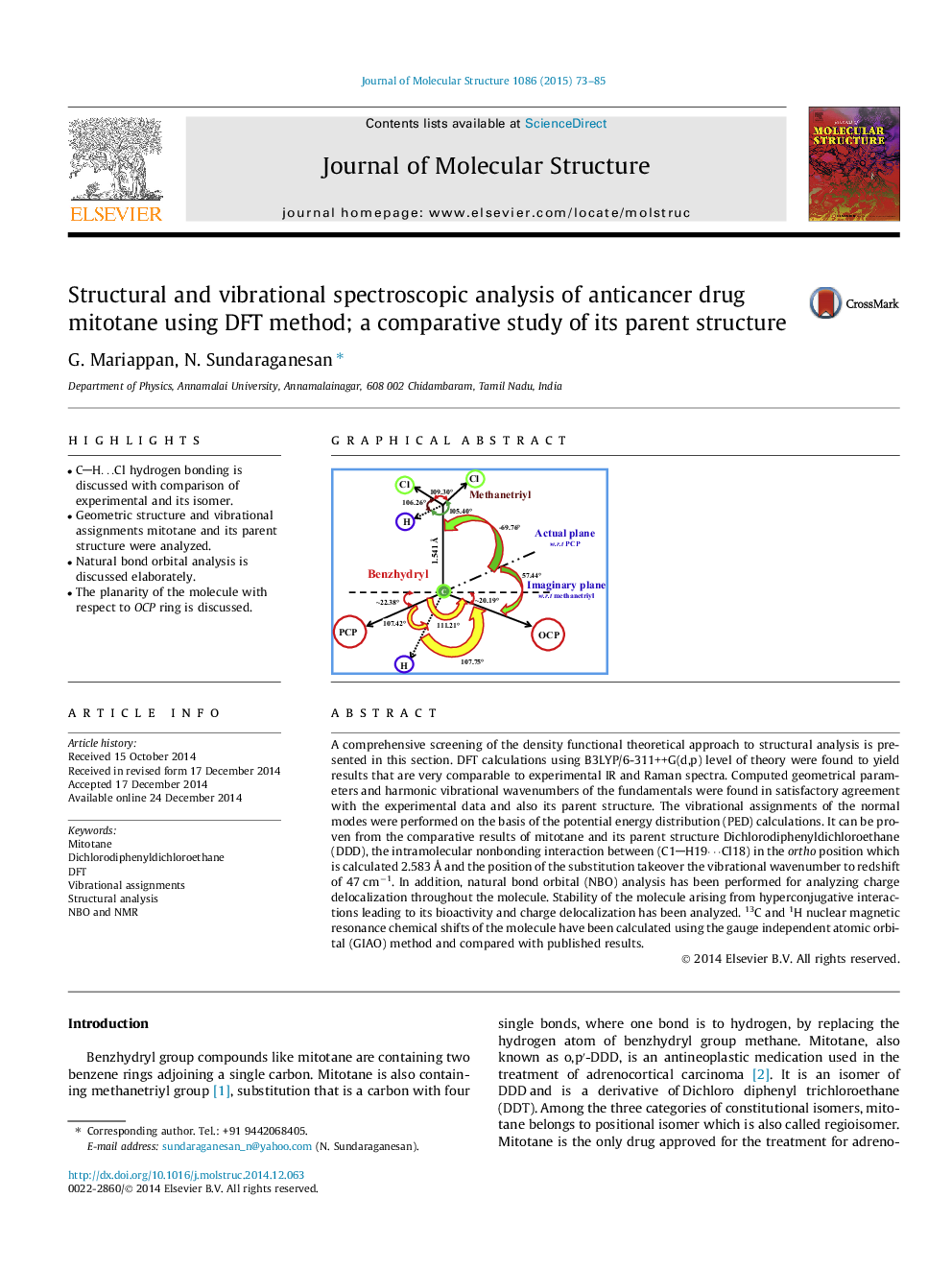
•CH…Cl hydrogen bonding is discussed with comparison of experimental and its isomer.•Geometric structure and vibrational assignments mitotane and its parent structure were analyzed.•Natural bond orbital analysis is discussed elaborately.•The planarity of the molecule with respect to OCP ring is discussed.
A comprehensive screening of the density functional theoretical approach to structural analysis is presented in this section. DFT calculations using B3LYP/6-311++G(d,p) level of theory were found to yield results that are very comparable to experimental IR and Raman spectra. Computed geometrical parameters and harmonic vibrational wavenumbers of the fundamentals were found in satisfactory agreement with the experimental data and also its parent structure. The vibrational assignments of the normal modes were performed on the basis of the potential energy distribution (PED) calculations. It can be proven from the comparative results of mitotane and its parent structure Dichlorodiphenyldichloroethane (DDD), the intramolecular nonbonding interaction between (C1H19⋯Cl18) in the ortho position which is calculated 2.583 Å and the position of the substitution takeover the vibrational wavenumber to redshift of 47 cm−1. In addition, natural bond orbital (NBO) analysis has been performed for analyzing charge delocalization throughout the molecule. Stability of the molecule arising from hyperconjugative interactions leading to its bioactivity and charge delocalization has been analyzed. 13C and 1H nuclear magnetic resonance chemical shifts of the molecule have been calculated using the gauge independent atomic orbital (GIAO) method and compared with published results.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide