| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1404962 | Journal of Molecular Structure | 2015 | 7 Pages |
•Glass transition temperature increases with increase in heating rate.•Cl− anions enter into the voids of borate network at low concentration.•Cl− anions contribute to the network formation at high concentrations.•Prepared glasses have good thermal stability.
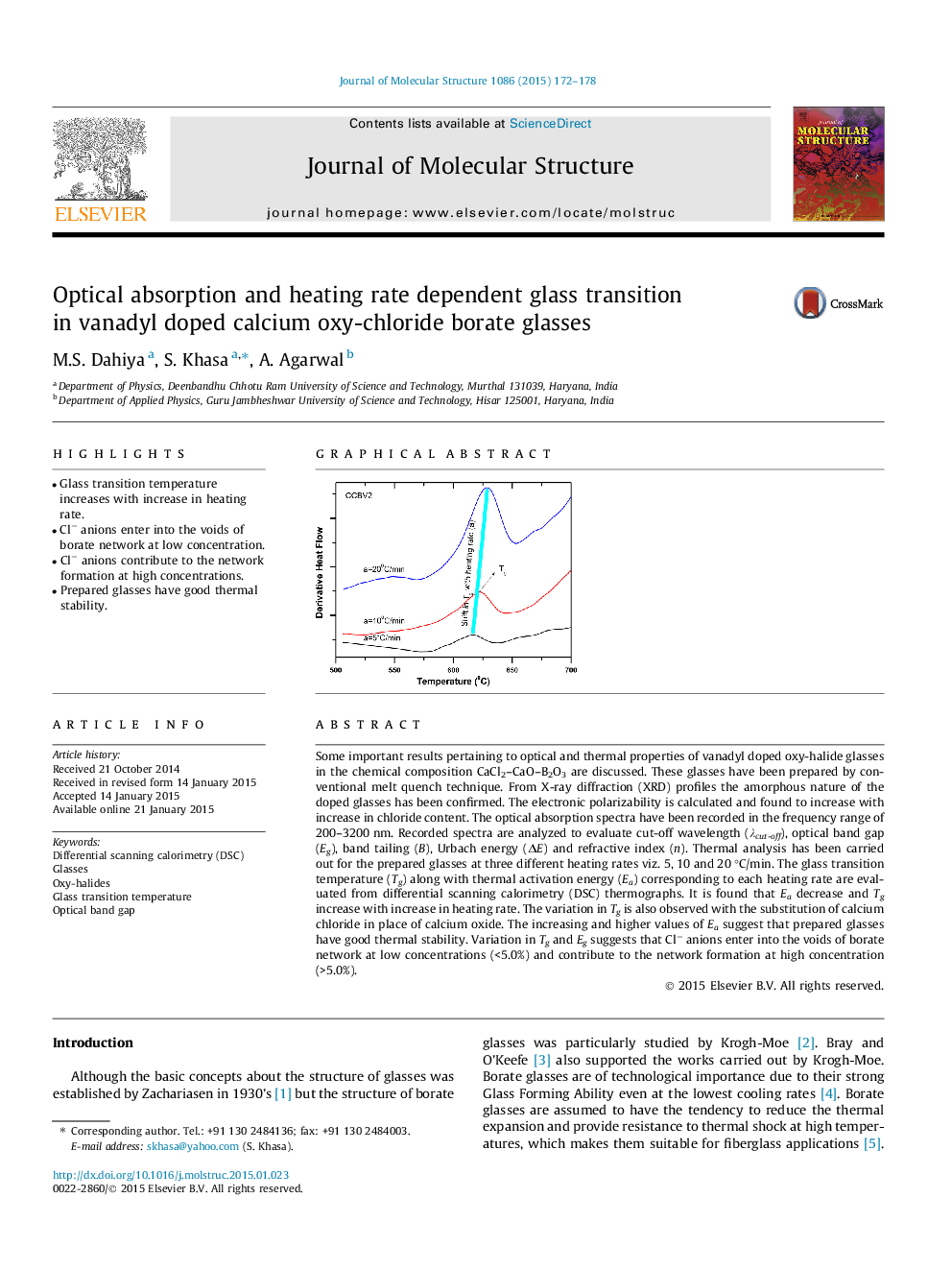
Some important results pertaining to optical and thermal properties of vanadyl doped oxy-halide glasses in the chemical composition CaCl2–CaO–B2O3 are discussed. These glasses have been prepared by conventional melt quench technique. From X-ray diffraction (XRD) profiles the amorphous nature of the doped glasses has been confirmed. The electronic polarizability is calculated and found to increase with increase in chloride content. The optical absorption spectra have been recorded in the frequency range of 200–3200 nm. Recorded spectra are analyzed to evaluate cut-off wavelength (λcut-off), optical band gap (Eg), band tailing (B), Urbach energy (ΔE) and refractive index (n). Thermal analysis has been carried out for the prepared glasses at three different heating rates viz. 5, 10 and 20 °C/min. The glass transition temperature (Tg) along with thermal activation energy (Ea) corresponding to each heating rate are evaluated from differential scanning calorimetry (DSC) thermographs. It is found that Ea decrease and Tg increase with increase in heating rate. The variation in Tg is also observed with the substitution of calcium chloride in place of calcium oxide. The increasing and higher values of Ea suggest that prepared glasses have good thermal stability. Variation in Tg and Eg suggests that Cl− anions enter into the voids of borate network at low concentrations (<5.0%) and contribute to the network formation at high concentration (>5.0%).
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide