| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1404963 | Journal of Molecular Structure | 2015 | 11 Pages |
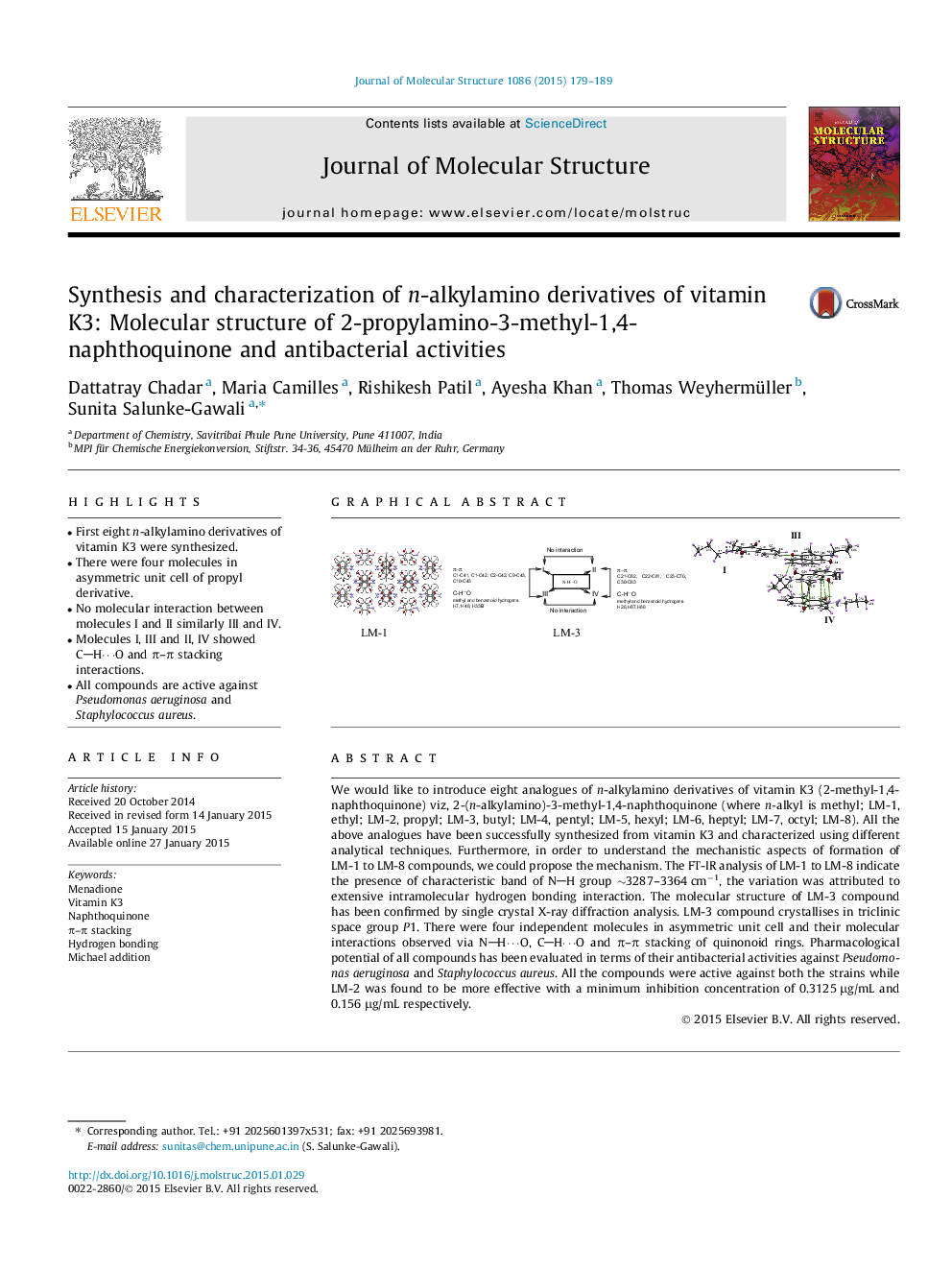
•First eight n-alkylamino derivatives of vitamin K3 were synthesized.•There were four molecules in asymmetric unit cell of propyl derivative.•No molecular interaction between molecules I and II similarly III and IV.•Molecules I, III and II, IV showed CH⋯O and π–π stacking interactions.•All compounds are active against Pseudomonas aeruginosa and Staphylococcus aureus.
We would like to introduce eight analogues of n-alkylamino derivatives of vitamin K3 (2-methyl-1,4-naphthoquinone) viz, 2-(n-alkylamino)-3-methyl-1,4-naphthoquinone (where n-alkyl is methyl; LM-1, ethyl; LM-2, propyl; LM-3, butyl; LM-4, pentyl; LM-5, hexyl; LM-6, heptyl; LM-7, octyl; LM-8). All the above analogues have been successfully synthesized from vitamin K3 and characterized using different analytical techniques. Furthermore, in order to understand the mechanistic aspects of formation of LM-1 to LM-8 compounds, we could propose the mechanism. The FT-IR analysis of LM-1 to LM-8 indicate the presence of characteristic band of NH group ∼3287–3364 cm−1, the variation was attributed to extensive intramolecular hydrogen bonding interaction. The molecular structure of LM-3 compound has been confirmed by single crystal X-ray diffraction analysis. LM-3 compound crystallises in triclinic space group P1. There were four independent molecules in asymmetric unit cell and their molecular interactions observed via NH⋯O, CH⋯O and π–π stacking of quinonoid rings. Pharmacological potential of all compounds has been evaluated in terms of their antibacterial activities against Pseudomonas aeruginosa and Staphylococcus aureus. All the compounds were active against both the strains while LM-2 was found to be more effective with a minimum inhibition concentration of 0.3125 μg/mL and 0.156 μg/mL respectively.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide