| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1405036 | Journal of Molecular Structure | 2014 | 4 Pages |
•Four Ni(II) ions in this complex possess two types of hybridization (dsp2 and sp3d2).•Both hydroxyl hydrogens of two oxime groups in EnH2 ligand are lost during the reaction.•Niether inter- nor intra-hydrogen bond is formed.•The obtained complex has a three-bladed propeller-like structure.
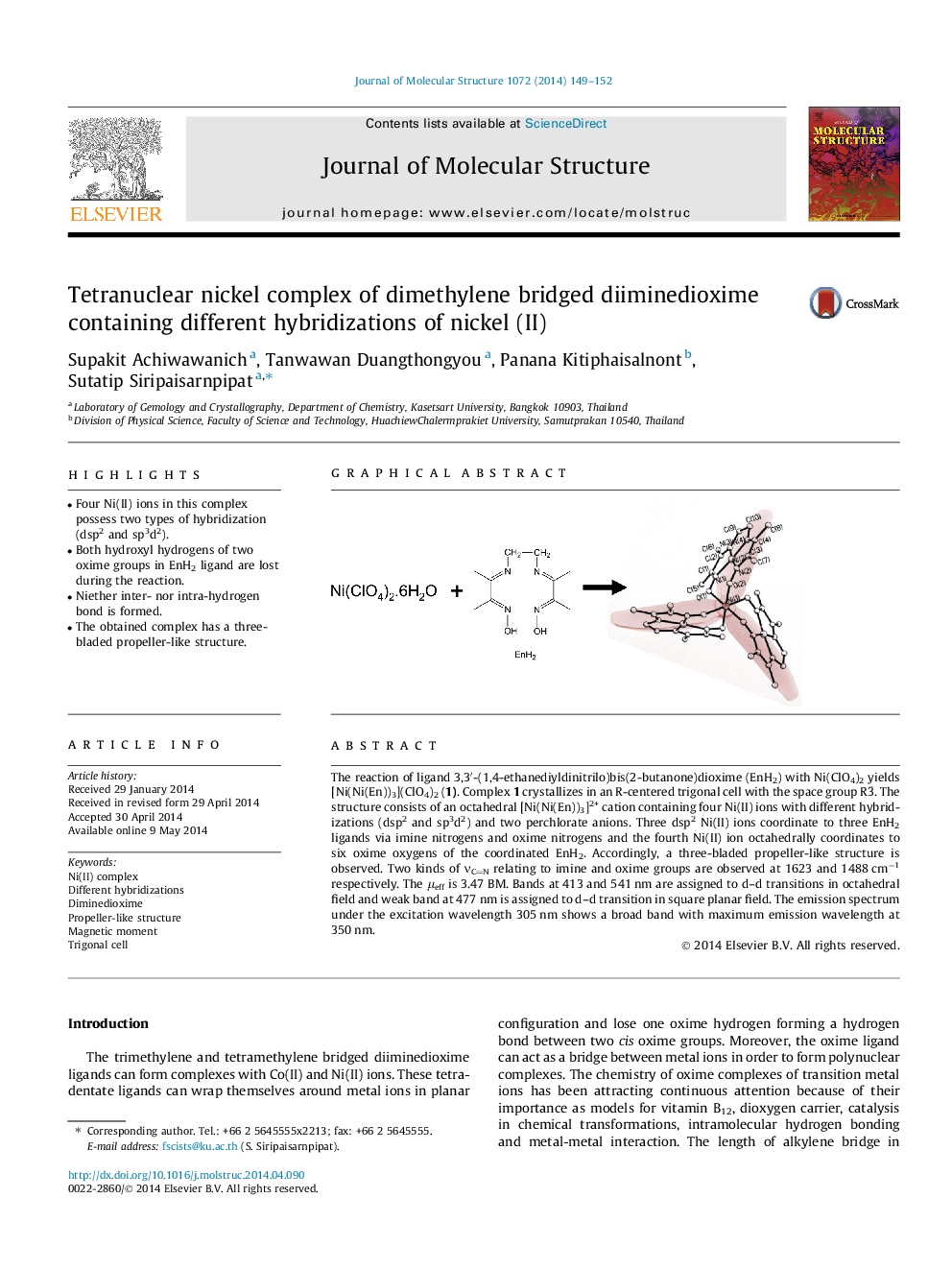
The reaction of ligand 3,3′-(1,4-ethanediyldinitrilo)bis(2-butanone)dioxime (EnH2) with Ni(ClO4)2 yields [Ni(Ni(En))3](ClO4)2 (1). Complex 1 crystallizes in an R-centered trigonal cell with the space group R3¯. The structure consists of an octahedral [Ni(Ni(En))3]2+ cation containing four Ni(II) ions with different hybridizations (dsp2 and sp3d2) and two perchlorate anions. Three dsp2 Ni(II) ions coordinate to three EnH2 ligands via imine nitrogens and oxime nitrogens and the fourth Ni(II) ion octahedrally coordinates to six oxime oxygens of the coordinated EnH2. Accordingly, a three-bladed propeller-like structure is observed. Two kinds of νCN relating to imine and oxime groups are observed at 1623 and 1488 cm−1 respectively. The μeff is 3.47 BM. Bands at 413 and 541 nm are assigned to d–d transitions in octahedral field and weak band at 477 nm is assigned to d–d transition in square planar field. The emission spectrum under the excitation wavelength 305 nm shows a broad band with maximum emission wavelength at 350 nm.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide