| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1405096 | Journal of Molecular Structure | 2014 | 7 Pages |
•Three d10 metal coordination polymers have been characterized.•Effect of dicarboxylates on the structure of complexes was discussed.•TG analysis confirms the stability of the coordination polymers.•Luminescence properties of complexes have been investigated.
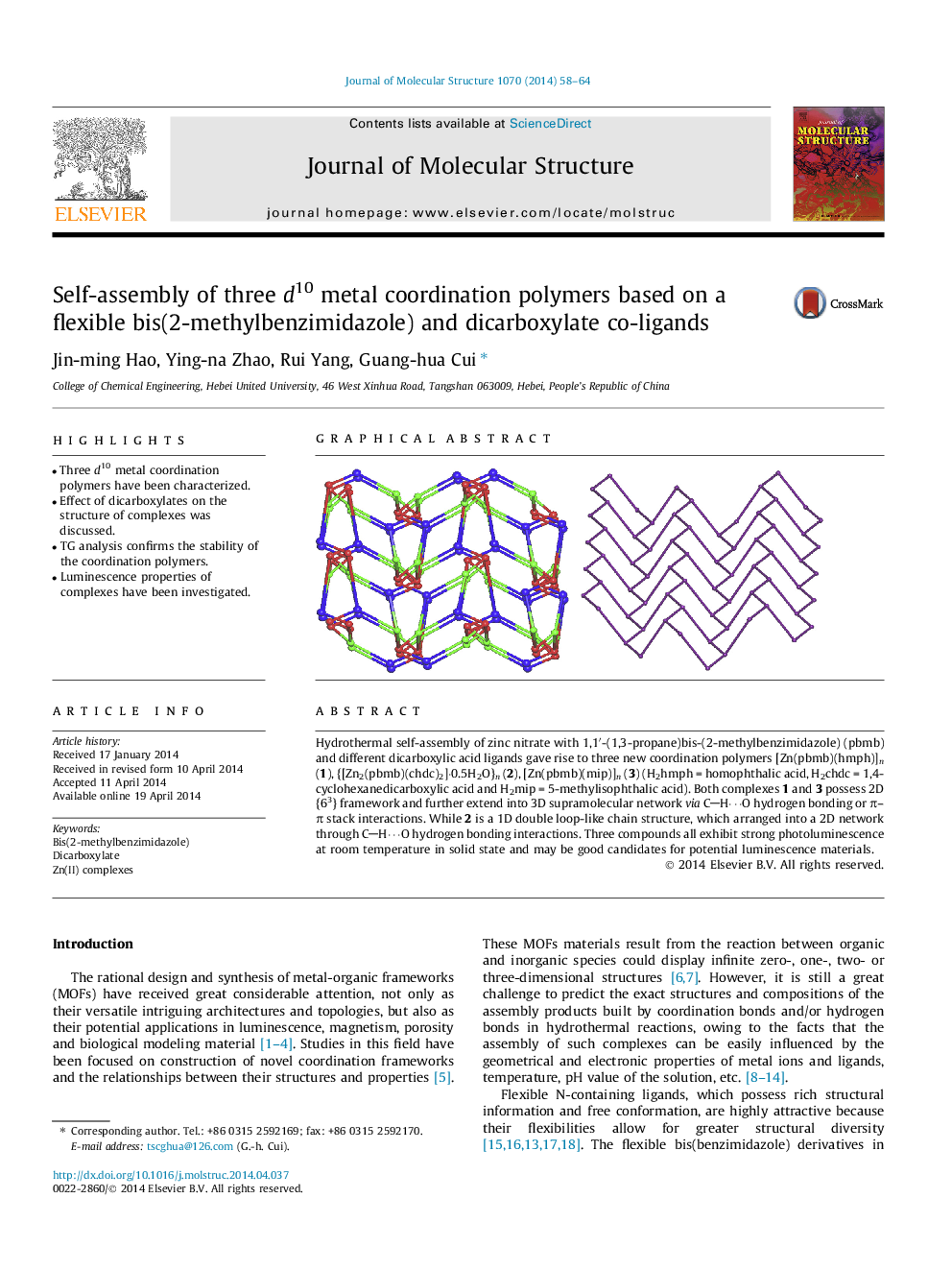
Hydrothermal self-assembly of zinc nitrate with 1,1′-(1,3-propane)bis-(2-methylbenzimidazole) (pbmb) and different dicarboxylic acid ligands gave rise to three new coordination polymers [Zn(pbmb)(hmph)]n (1), {[Zn2(pbmb)(chdc)2]⋅0.5H2O}n (2), [Zn(pbmb)(mip)]n (3) (H2hmph = homophthalic acid, H2chdc = 1,4-cyclohexanedicarboxylic acid and H2mip = 5-methylisophthalic acid). Both complexes 1 and 3 possess 2D {63} framework and further extend into 3D supramolecular network via CH⋯O hydrogen bonding or π–π stack interactions. While 2 is a 1D double loop-like chain structure, which arranged into a 2D network through CH⋯O hydrogen bonding interactions. Three compounds all exhibit strong photoluminescence at room temperature in solid state and may be good candidates for potential luminescence materials.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide