| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1405222 | Journal of Molecular Structure | 2014 | 4 Pages |
•Stability of the Na+ – sodium ionophore III complex was determined.•Quantum mechanical DFT calculations were carried out.•Structures of the nonhydrated and hydrated cationic complexes were predicted.
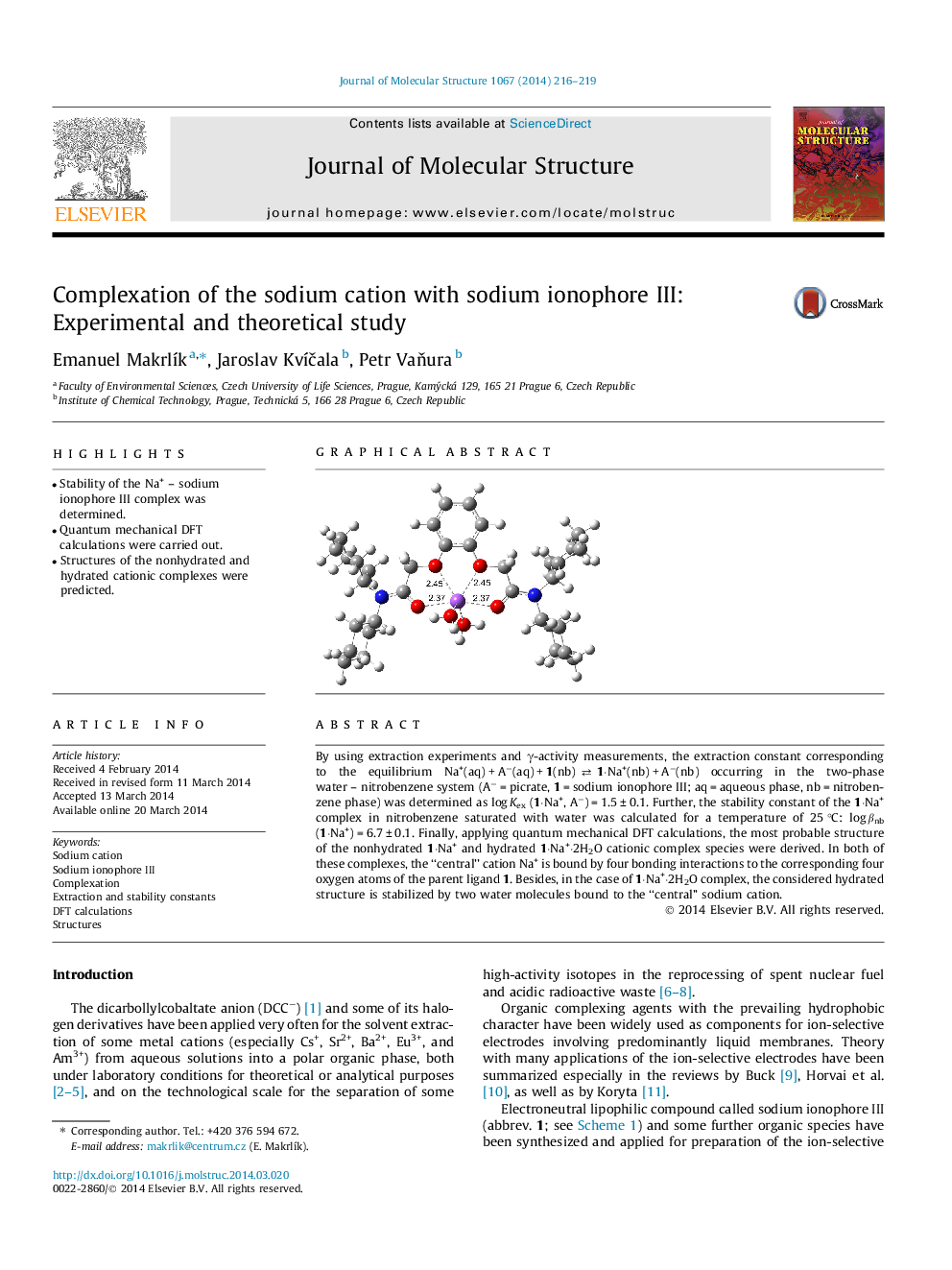
By using extraction experiments and γ-activity measurements, the extraction constant corresponding to the equilibrium Na+(aq) + A−(aq) + 1(nb) ⇄⇄ 1·Na+(nb) + A−(nb) occurring in the two-phase water – nitrobenzene system (A− = picrate, 1 = sodium ionophore III; aq = aqueous phase, nb = nitrobenzene phase) was determined as log Kex (1·Na+, A−) = 1.5 ± 0.1. Further, the stability constant of the 1·Na+ complex in nitrobenzene saturated with water was calculated for a temperature of 25 °C: log βnb (1·Na+) = 6.7 ± 0.1. Finally, applying quantum mechanical DFT calculations, the most probable structure of the nonhydrated 1·Na+ and hydrated 1·Na+·2H2O cationic complex species were derived. In both of these complexes, the “central” cation Na+ is bound by four bonding interactions to the corresponding four oxygen atoms of the parent ligand 1. Besides, in the case of 1·Na+·2H2O complex, the considered hydrated structure is stabilized by two water molecules bound to the “central” sodium cation.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide