| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1405309 | Journal of Molecular Structure | 2013 | 9 Pages |
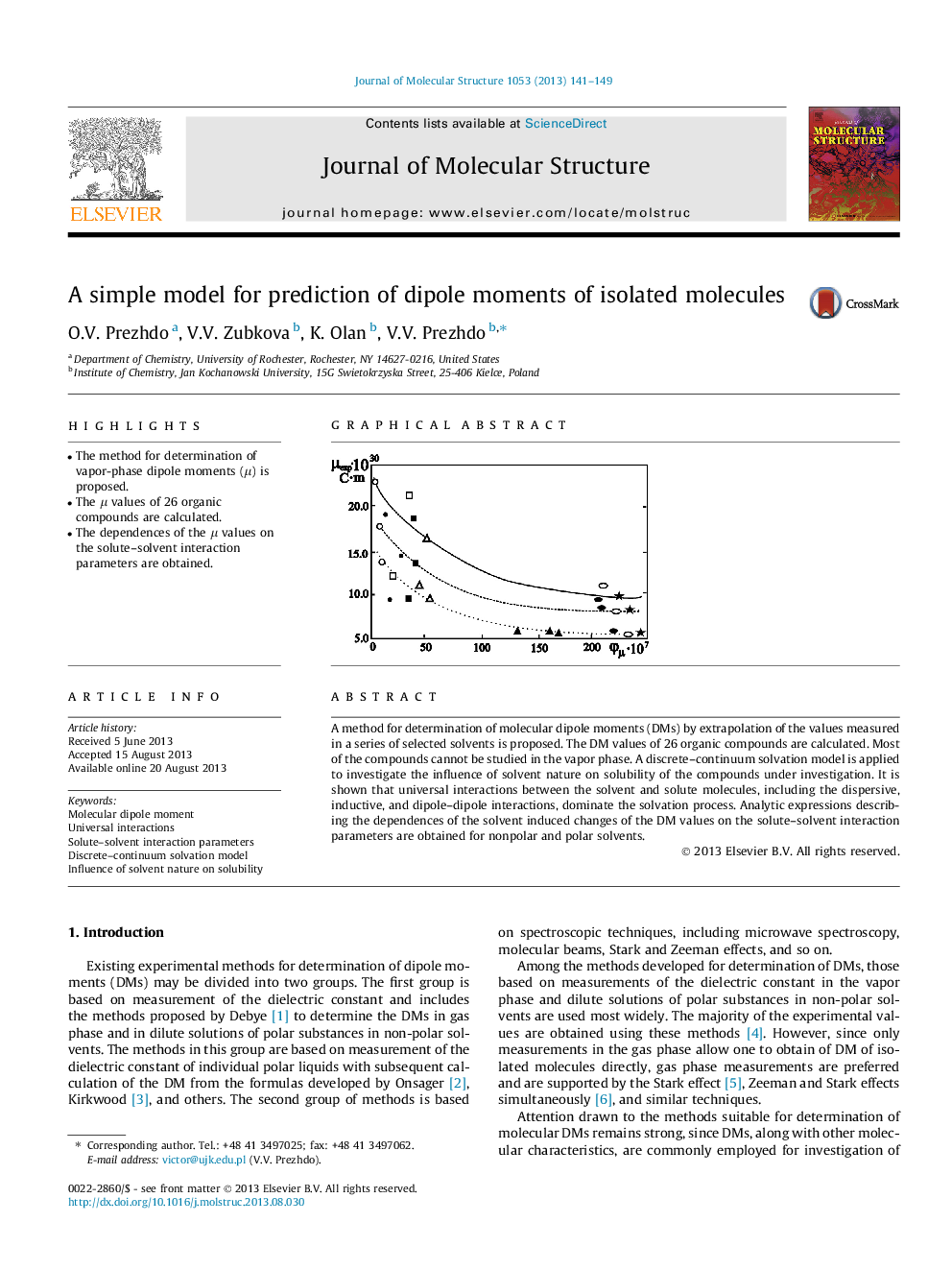
•The method for determination of vapor-phase dipole moments (μ) is proposed.•The μ values of 26 organic compounds are calculated.•The dependences of the μ values on the solute–solvent interaction parameters are obtained.
A method for determination of molecular dipole moments (DMs) by extrapolation of the values measured in a series of selected solvents is proposed. The DM values of 26 organic compounds are calculated. Most of the compounds cannot be studied in the vapor phase. A discrete–continuum solvation model is applied to investigate the influence of solvent nature on solubility of the compounds under investigation. It is shown that universal interactions between the solvent and solute molecules, including the dispersive, inductive, and dipole–dipole interactions, dominate the solvation process. Analytic expressions describing the dependences of the solvent induced changes of the DM values on the solute–solvent interaction parameters are obtained for nonpolar and polar solvents.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide