| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1405376 | Journal of Molecular Structure | 2015 | 8 Pages |
•Azonaphtharylamide pigments having 2- and 7-substituents were studied.•The pigments exhibited a hyperchromic effect and no bathochromic shift compared with the 7-unsubstituted counterparts.•Light and heat fastness of the pigments outperformed those of the counterparts.•The pigments are expected to form a new subclass of the azo pigments.
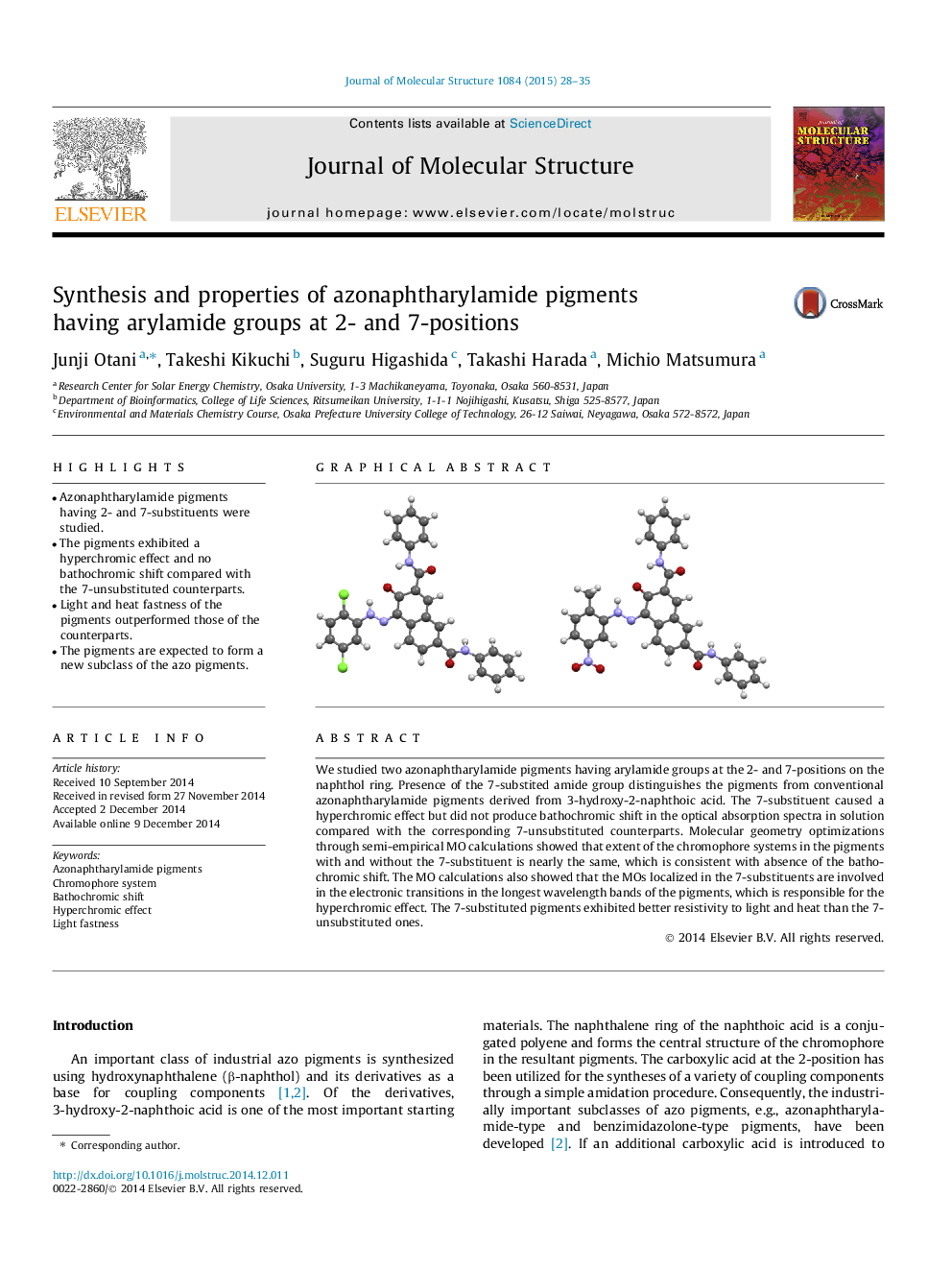
We studied two azonaphtharylamide pigments having arylamide groups at the 2- and 7-positions on the naphthol ring. Presence of the 7-substited amide group distinguishes the pigments from conventional azonaphtharylamide pigments derived from 3-hydroxy-2-naphthoic acid. The 7-substituent caused a hyperchromic effect but did not produce bathochromic shift in the optical absorption spectra in solution compared with the corresponding 7-unsubstituted counterparts. Molecular geometry optimizations through semi-empirical MO calculations showed that extent of the chromophore systems in the pigments with and without the 7-substituent is nearly the same, which is consistent with absence of the bathochromic shift. The MO calculations also showed that the MOs localized in the 7-substituents are involved in the electronic transitions in the longest wavelength bands of the pigments, which is responsible for the hyperchromic effect. The 7-substituted pigments exhibited better resistivity to light and heat than the 7-unsubstituted ones.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide