| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1405377 | Journal of Molecular Structure | 2015 | 10 Pages |
•Metal complexes are synthesized from Schiff’s base ligand.•Schiff’s base metal complexes are characterized by spectral analysis.•Kinetic parameters have been calculated.•Molecular modeling of the compounds have been calculated.•Biological activities on different bacteria and fungal were examined.
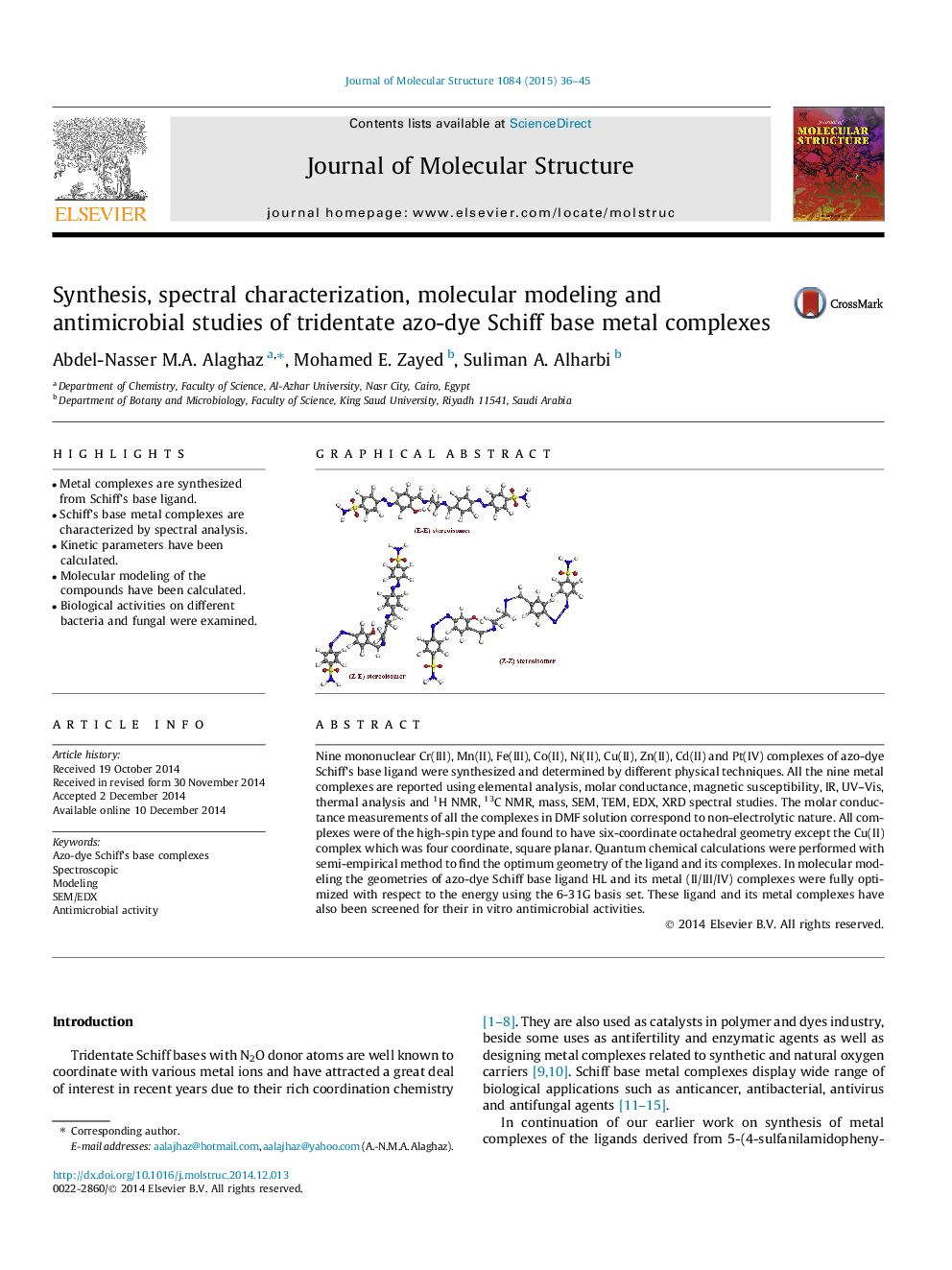
Nine mononuclear Cr(III), Mn(II), Fe(III), Co(II), Ni(II), Cu(II), Zn(II), Cd(II) and Pt(IV) complexes of azo-dye Schiff’s base ligand were synthesized and determined by different physical techniques. All the nine metal complexes are reported using elemental analysis, molar conductance, magnetic susceptibility, IR, UV–Vis, thermal analysis and 1H NMR, 13C NMR, mass, SEM, TEM, EDX, XRD spectral studies. The molar conductance measurements of all the complexes in DMF solution correspond to non-electrolytic nature. All complexes were of the high-spin type and found to have six-coordinate octahedral geometry except the Cu(II) complex which was four coordinate, square planar. Quantum chemical calculations were performed with semi-empirical method to find the optimum geometry of the ligand and its complexes. In molecular modeling the geometries of azo-dye Schiff base ligand HL and its metal (II/III/IV) complexes were fully optimized with respect to the energy using the 6-31G basis set. These ligand and its metal complexes have also been screened for their in vitro antimicrobial activities.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide