| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1405483 | Journal of Molecular Structure | 2014 | 7 Pages |
•Infrared spectra of gas and Raman spectra of liquid isopropyl isocyanide were recorded.•Vibrational assignments have been obtained for isopropyl isocyanide.•r0 structural parameters were determined for the isopropyl isocyanide.
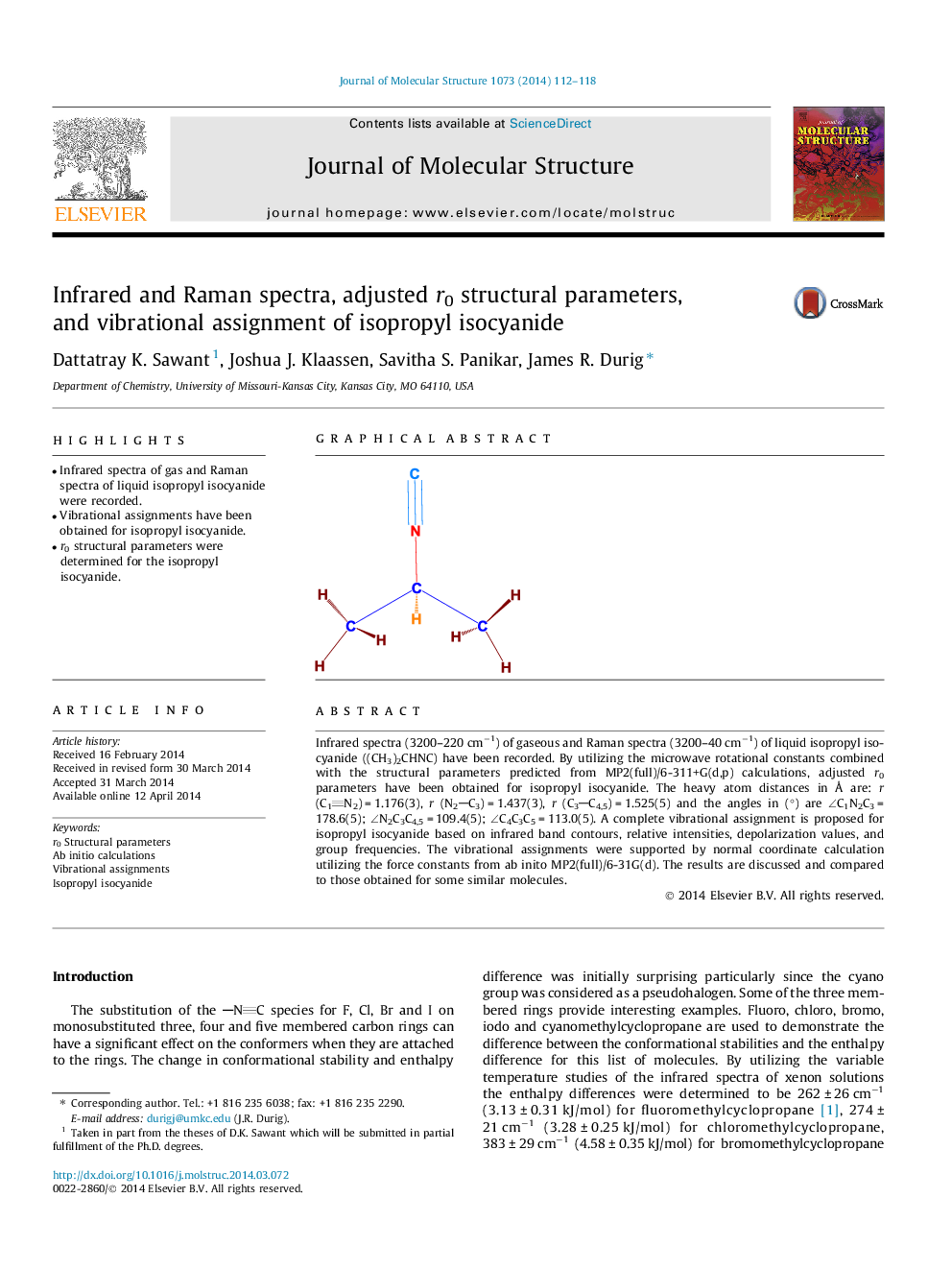
Infrared spectra (3200–220 cm−1) of gaseous and Raman spectra (3200–40 cm−1) of liquid isopropyl isocyanide ((CH3)2CHNC) have been recorded. By utilizing the microwave rotational constants combined with the structural parameters predicted from MP2(full)/6-311+G(d,p) calculations, adjusted r0 parameters have been obtained for isopropyl isocyanide. The heavy atom distances in Å are: r (C1N2) = 1.176(3), r (N2C3) = 1.437(3), r (C3C4,5) = 1.525(5) and the angles in (°) are ∠∠C1N2C3 = 178.6(5); ∠∠N2C3C4,5 = 109.4(5); ∠∠C4C3C5 = 113.0(5). A complete vibrational assignment is proposed for isopropyl isocyanide based on infrared band contours, relative intensities, depolarization values, and group frequencies. The vibrational assignments were supported by normal coordinate calculation utilizing the force constants from ab inito MP2(full)/6-31G(d). The results are discussed and compared to those obtained for some similar molecules.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide