| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1405556 | Journal of Molecular Structure | 2014 | 8 Pages |
•A new polydentate ligand and five complexes were synthesized successfully.•Complexes 1–3 show different π–π interactions.•Anion–π interactions were observed in 4 and 5.•Supramolecular ladders and chains were observed in the packing structures of 4 and 5.•Non-covalent interactions play significant roles in the stabilization of 1–5.
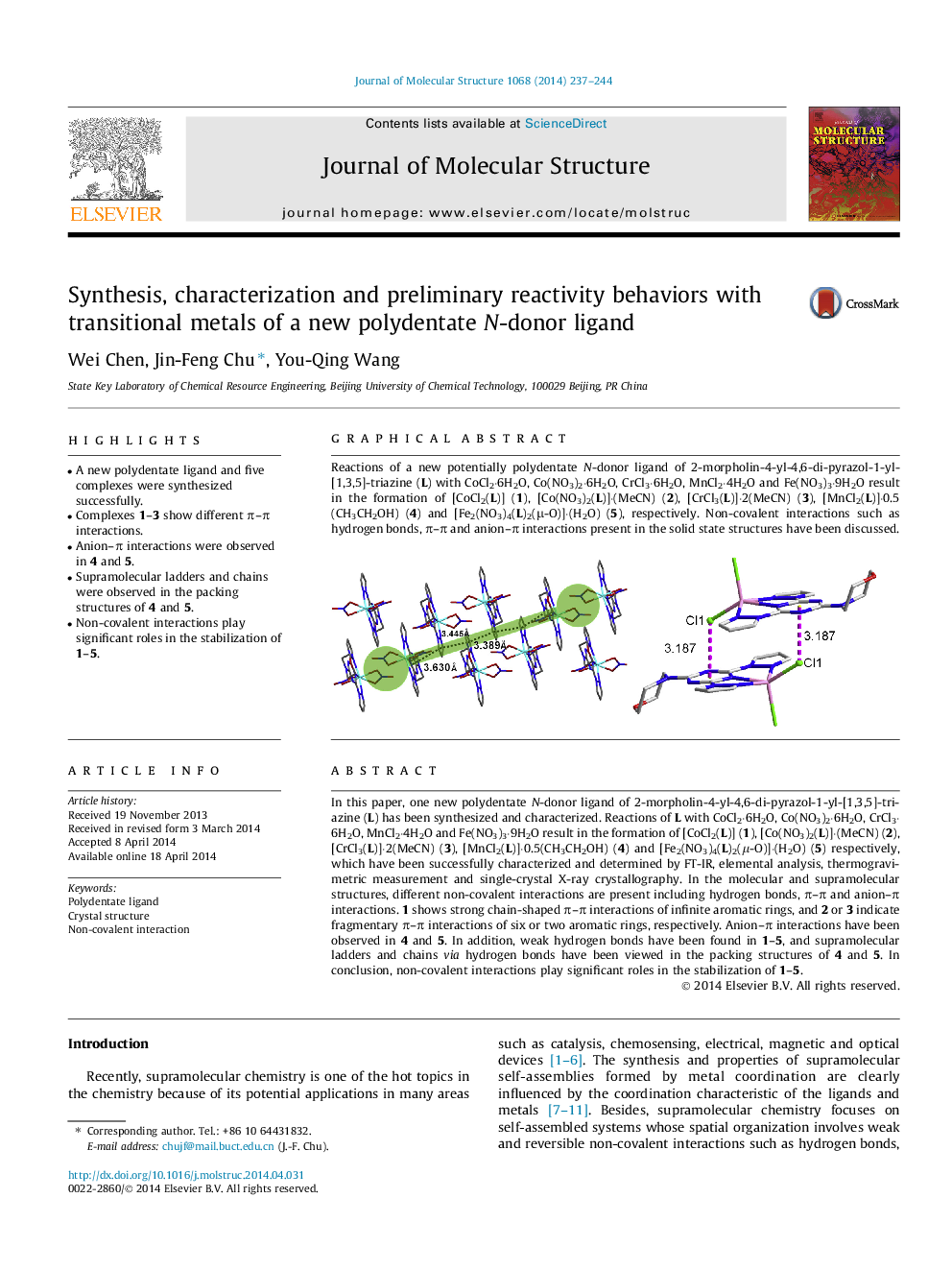
In this paper, one new polydentate N-donor ligand of 2-morpholin-4-yl-4,6-di-pyrazol-1-yl-[1,3,5]-triazine (L) has been synthesized and characterized. Reactions of L with CoCl2·6H2O, Co(NO3)2·6H2O, CrCl3·6H2O, MnCl2·4H2O and Fe(NO3)3·9H2O result in the formation of [CoCl2(L)] (1), [Co(NO3)2(L)]·(MeCN) (2), [CrCl3(L)]·2(MeCN) (3), [MnCl2(L)]·0.5(CH3CH2OH) (4) and [Fe2(NO3)4(L)2(μ-O)]·(H2O) (5) respectively, which have been successfully characterized and determined by FT-IR, elemental analysis, thermogravimetric measurement and single-crystal X-ray crystallography. In the molecular and supramolecular structures, different non-covalent interactions are present including hydrogen bonds, π–π and anion–π interactions. 1 shows strong chain-shaped π–π interactions of infinite aromatic rings, and 2 or 3 indicate fragmentary π–π interactions of six or two aromatic rings, respectively. Anion–π interactions have been observed in 4 and 5. In addition, weak hydrogen bonds have been found in 1–5, and supramolecular ladders and chains via hydrogen bonds have been viewed in the packing structures of 4 and 5. In conclusion, non-covalent interactions play significant roles in the stabilization of 1–5.
Graphical abstractReactions of a new potentially polydentate N-donor ligand of 2-morpholin-4-yl-4,6-di-pyrazol-1-yl-[1,3,5]-triazine (L) with CoCl2·6H2O, Co(NO3)2·6H2O, CrCl3·6H2O, MnCl2·4H2O and Fe(NO3)3·9H2O result in the formation of [CoCl2(L)] (1), [Co(NO3)2(L)]·(MeCN) (2), [CrCl3(L)]·2(MeCN) (3), [MnCl2(L)]·0.5(CH3CH2OH) (4) and [Fe2(NO3)4(L)2(μ-O)]·(H2O) (5), respectively. Non-covalent interactions such as hydrogen bonds, π–π and anion–π interactions present in the solid state structures have been discussed.Figure optionsDownload full-size imageDownload as PowerPoint slide