| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1405766 | Journal of Molecular Structure | 2014 | 9 Pages |
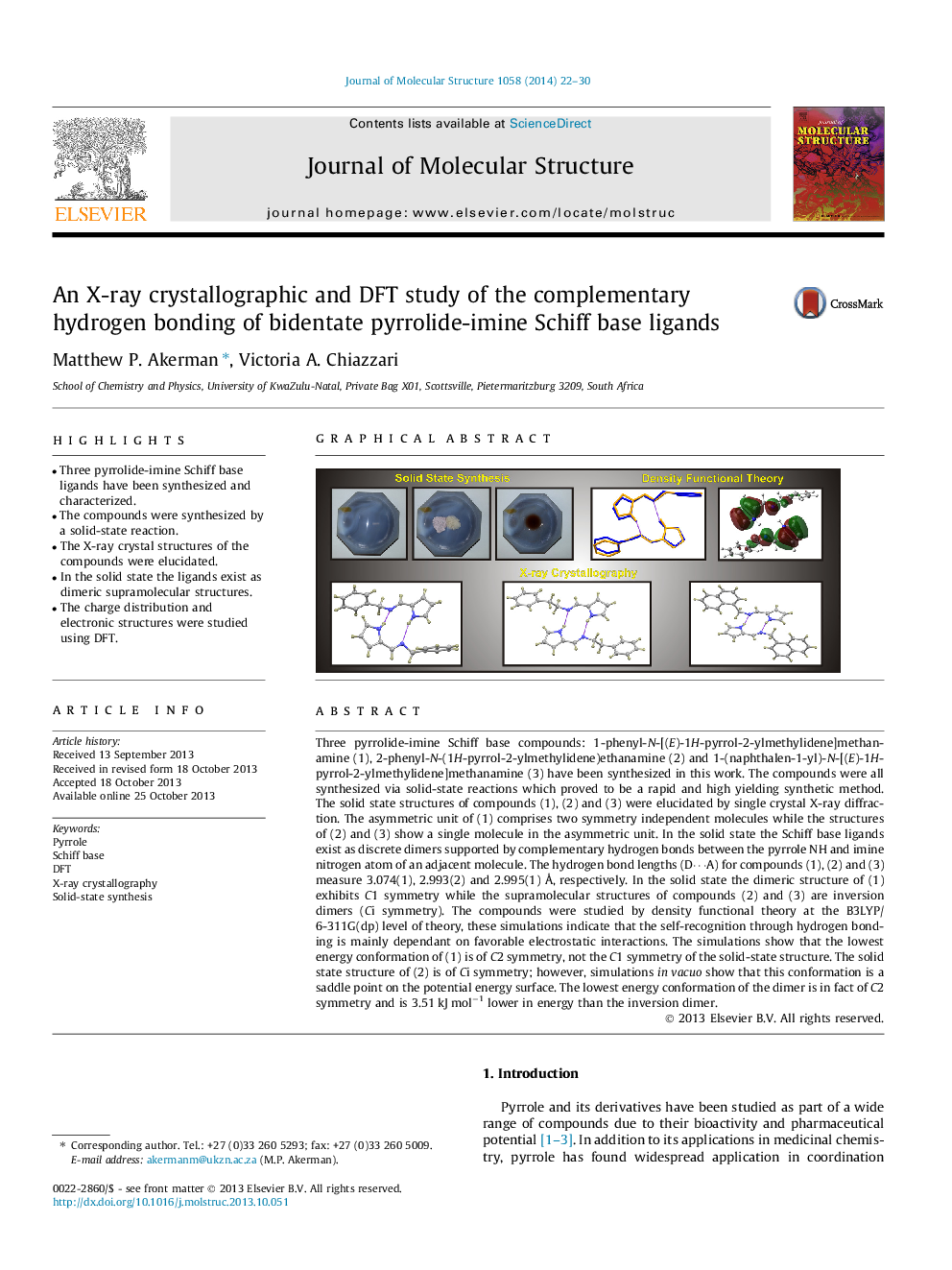
•Three pyrrolide-imine Schiff base ligands have been synthesized and characterized.•The compounds were synthesized by a solid-state reaction.•The X-ray crystal structures of the compounds were elucidated.•In the solid state the ligands exist as dimeric supramolecular structures.•The charge distribution and electronic structures were studied using DFT.
Three pyrrolide-imine Schiff base compounds: 1-phenyl-N-[(E)-1H-pyrrol-2-ylmethylidene]methanamine (1), 2-phenyl-N-(1H-pyrrol-2-ylmethylidene)ethanamine (2) and 1-(naphthalen-1-yl)-N-[(E)-1H-pyrrol-2-ylmethylidene]methanamine (3) have been synthesized in this work. The compounds were all synthesized via solid-state reactions which proved to be a rapid and high yielding synthetic method. The solid state structures of compounds (1), (2) and (3) were elucidated by single crystal X-ray diffraction. The asymmetric unit of (1) comprises two symmetry independent molecules while the structures of (2) and (3) show a single molecule in the asymmetric unit. In the solid state the Schiff base ligands exist as discrete dimers supported by complementary hydrogen bonds between the pyrrole NH and imine nitrogen atom of an adjacent molecule. The hydrogen bond lengths (D⋯A) for compounds (1), (2) and (3) measure 3.074(1), 2.993(2) and 2.995(1) Å, respectively. In the solid state the dimeric structure of (1) exhibits C1 symmetry while the supramolecular structures of compounds (2) and (3) are inversion dimers (Ci symmetry). The compounds were studied by density functional theory at the B3LYP/6-311G(dp) level of theory, these simulations indicate that the self-recognition through hydrogen bonding is mainly dependant on favorable electrostatic interactions. The simulations show that the lowest energy conformation of (1) is of C2 symmetry, not the C1 symmetry of the solid-state structure. The solid state structure of (2) is of Ci symmetry; however, simulations in vacuo show that this conformation is a saddle point on the potential energy surface. The lowest energy conformation of the dimer is in fact of C2 symmetry and is 3.51 kJ mol−1 lower in energy than the inversion dimer.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide