| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1405775 | Journal of Molecular Structure | 2014 | 8 Pages |
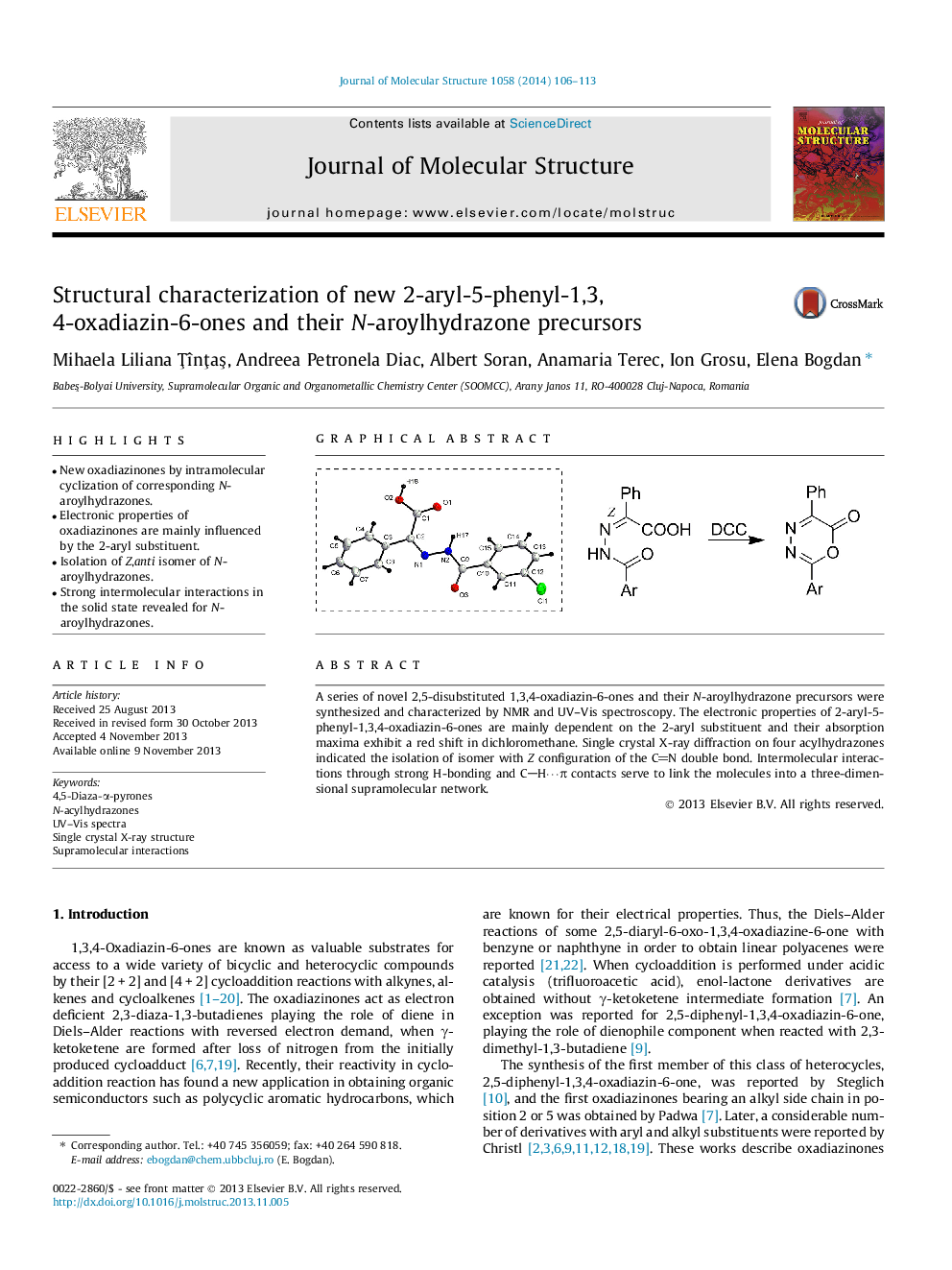
•New oxadiazinones by intramolecular cyclization of corresponding N-aroylhydrazones.•Electronic properties of oxadiazinones are mainly influenced by the 2-aryl substituent.•Isolation of Z,anti isomer of N-aroylhydrazones.•Strong intermolecular interactions in the solid state revealed for N-aroylhydrazones.
A series of novel 2,5-disubstituted 1,3,4-oxadiazin-6-ones and their N-aroylhydrazone precursors were synthesized and characterized by NMR and UV–Vis spectroscopy. The electronic properties of 2-aryl-5-phenyl-1,3,4-oxadiazin-6-ones are mainly dependent on the 2-aryl substituent and their absorption maxima exhibit a red shift in dichloromethane. Single crystal X-ray diffraction on four acylhydrazones indicated the isolation of isomer with Z configuration of the CN double bond. Intermolecular interactions through strong H-bonding and CH⋯π contacts serve to link the molecules into a three-dimensional supramolecular network.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide