| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1405786 | Journal of Molecular Structure | 2014 | 8 Pages |
•Pogostone and two novel dimers of Pogostone were prepared simultaneously.•Pogostone was first charactered by X-ray diffraction.•Two diastereomers were elucidated by 1H and 13C NMR, COSY, HMQC and HMBC together with X-ray diffraction.•X-ray results indicated there solely existed H-bond intramolecular interactions in the two compounds.
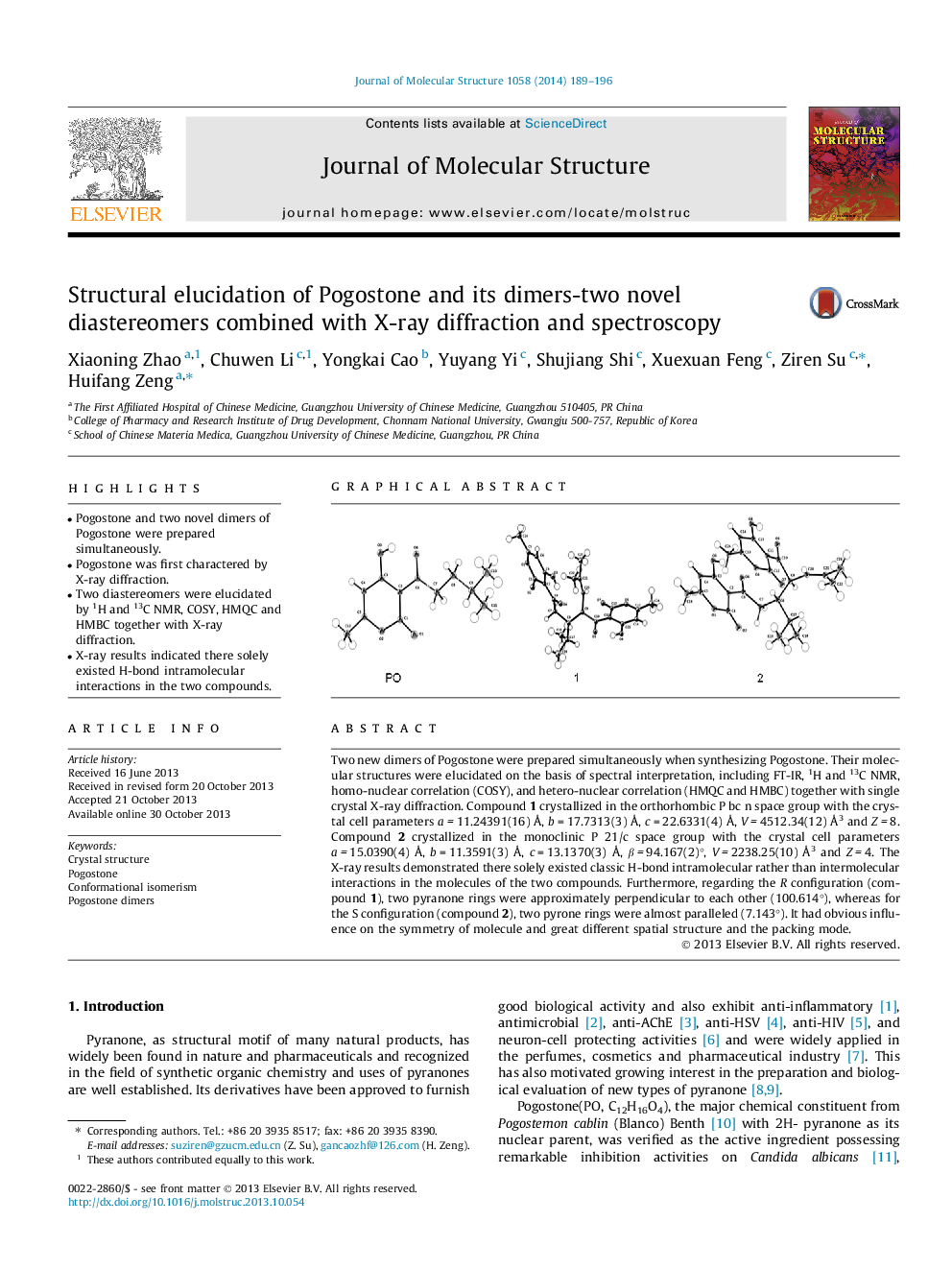
Two new dimers of Pogostone were prepared simultaneously when synthesizing Pogostone. Their molecular structures were elucidated on the basis of spectral interpretation, including FT-IR, 1H and 13C NMR, homo-nuclear correlation (COSY), and hetero-nuclear correlation (HMQC and HMBC) together with single crystal X-ray diffraction. Compound 1 crystallized in the orthorhombic P bc n space group with the crystal cell parameters a = 11.24391(16) Å, b = 17.7313(3) Å, c = 22.6331(4) Å, V = 4512.34(12) Å3 and Z = 8. Compound 2 crystallized in the monoclinic P 21/c space group with the crystal cell parameters a = 15.0390(4) Å, b = 11.3591(3) Å, c = 13.1370(3) Å, β = 94.167(2)°, V = 2238.25(10) Å3 and Z = 4. The X-ray results demonstrated there solely existed classic H-bond intramolecular rather than intermolecular interactions in the molecules of the two compounds. Furthermore, regarding the R configuration (compound 1), two pyranone rings were approximately perpendicular to each other (100.614°), whereas for the S configuration (compound 2), two pyrone rings were almost paralleled (7.143°). It had obvious influence on the symmetry of molecule and great different spatial structure and the packing mode.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide