| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1405846 | Journal of Molecular Structure | 2013 | 7 Pages |
•A novel water-anion-(carboxyl oxygen) tape.•A new mode of interaction among water molecules, anions and carboxylate oxygen atoms.•Intense blue luminescence in the solid-state at room temperature.•1 shows good catalytic activity for the degradation of methyl orange dye.
Unusual tetramers and water–nitrate clusters have been observed in a mononuclear zinc(II) complex of [Zn(4-cpa)(phen)2(H2O)]·(H2O)·(NO3)] (1), (4-Hcpa = 4-chlorophenoxyacetic acid, phen = 1,10-phenanthroline), which was synthesized under hydrothermal conditions and characterized by elemental analysis, IR spectroscopy, thermogravimetric analysis (TGA), powder X-ray diffraction, UV–vis absorption spectra and single-crystal X-ray diffraction. The crystal structure analysis of 1, reveals that the nitrate anions, water molecules and carboxylate oxygen atoms (O3) pack to form a one-dimensional infinite tape parallel to the c-axis. The uncoordinated carboxylate oxygen atoms (O3) of 4-cpa ligands, water molecules and nitrate anions interact via hydrogen bonds and extend 1 into a water–anion-cation tape, which are finally connected into a 3D supramolecular structure via π⋯π stacking interactions. Excitation (λex = 310 nm) and luminescence data observed at room temperature show that 1 emits bright blue fluorescence. Moreover, 1 has a remarkable activity for degradation of methyl orange in a photo-assisted Fenton-like process.
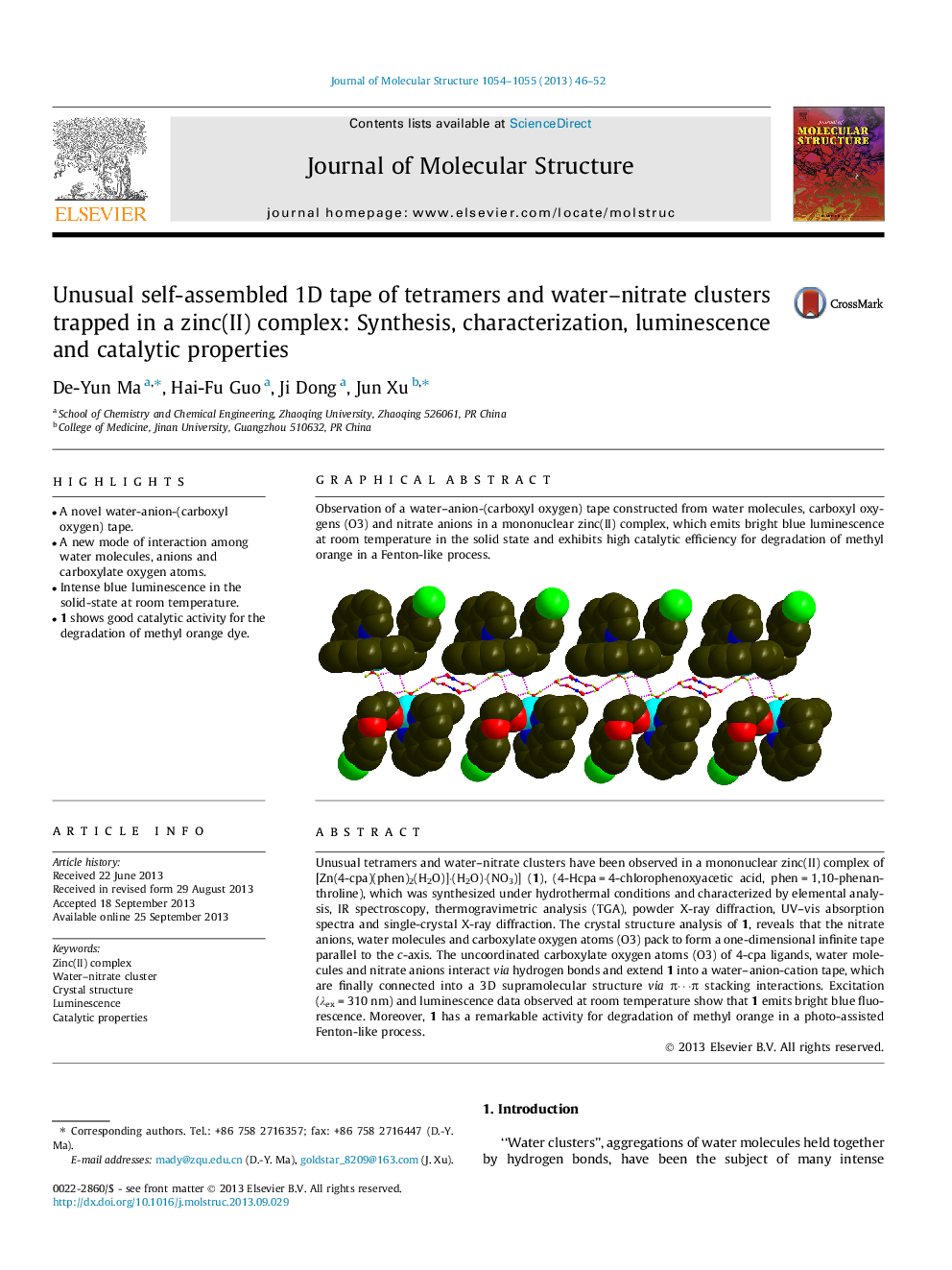
Graphical abstractObservation of a water–anion-(carboxyl oxygen) tape constructed from water molecules, carboxyl oxygens (O3) and nitrate anions in a mononuclear zinc(II) complex, which emits bright blue luminescence at room temperature in the solid state and exhibits high catalytic efficiency for degradation of methyl orange in a Fenton-like process.Figure optionsDownload full-size imageDownload as PowerPoint slide